Note from the Fluoride Action Network:
Molecular structure for Sulfoxaflor (see more information on its toxicity and USDA Shuts Down Data Collection on Honey Bees) and Sulfoxaflor: Documents.
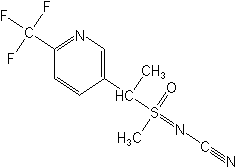
_________________________________________________________________
Online at https://www.federalregister.gov/documents/2019/10/25/2019-23384/sulfoxaflor-pesticide-tolerances
AGENCY:
Environmental Protection Agency (EPA).
ACTION:
Final rule.
SUMMARY:
This regulation establishes tolerances for residues of sulfoxaflor in or on rice grain, rice hulls, and avocados. Dow AgroSciences LLC requested these tolerances under the Federal Food, Drug, and Cosmetic Act (FFDCA).
DATES:
This regulation is effective October 25, 2019. Objections and requests for hearings must be received on or before December 24, 2019, and must be filed in accordance with the instructions provided in 40 CFR part 178 (see also Unit I.C. of the SUPPLEMENTARY INFORMATION).
ADDRESSES:
The docket for this action, identified by docket identification (ID) number EPA-HQ-OPP-2018-0599, is available at http://www.regulations.gov or at the Office of Pesticide Programs Regulatory Public Docket (OPP Docket) in the Environmental Protection Agency Docket Center (EPA/DC), West William Jefferson Clinton Bldg., Rm. 3334, 1301 Constitution Ave. NW, Washington, DC 20460-0001. The Public Reading Room is open from 8:30 a.m. to 4:30 p.m., Monday through Friday, excluding legal holidays. The telephone number for the Public Reading Room is (202) 566-1744, and the telephone number for the OPP Docket is (703) 305-5805. Please review the visitor instructions and additional information about the docket available at http://www.epa.gov/?dockets.
FOR FURTHER INFORMATION CONTACT:
Michael Goodis, Registration Division (7505P), Office of Pesticide Programs, Environmental Protection Agency, 1200 Pennsylvania Ave. NW, Washington, DC 20460-0001; main telephone number: (703) 305-7090; email address: RDFRNotices@epa.gov.
SUPPLEMENTARY INFORMATION:
I. General Information
A. Does this action apply to me?
You may be potentially affected by this action if you are an agricultural producer, food manufacturer, or pesticide manufacturer. The following list of North American Industrial Classification System (NAICS) codes is not intended to be exhaustive, but rather provides a guide to help readers determine whether this document applies to them. Potentially affected entities may include:
- Crop production (NAICS code 111).
- Animal production (NAICS code 112).
- Food manufacturing (NAICS code 311).
- Pesticide manufacturing (NAICS code 32532).
B. How can I get electronic access to other related information?
You may access a frequently updated electronic version of EPA’s tolerance regulations at 40 CFR part 180 through the Government Publishing Office’s e-CFR site at http://www.ecfr.gov/?cgi-bin/?text-idx??&?c=?ecfr&?tpl=?/?ecfrbrowse/?Title40/?40tab_?02.tpl.
C. How can I file an objection or hearing request?
Under FFDCA section 408(g), 21 U.S.C. 346a, any person may file an objection to any aspect of this regulation and may also request a hearing on those objections. You must file your objection or request a hearing on this regulation in accordance with the instructions provided in 40 CFR part 178. To ensure proper receipt by EPA, you must identify docket ID number EPA-HQ-OPP-2018-0599 in the subject line on the first page of your submission. All objections and requests for a hearing must be in writing and must be received by the Hearing Clerk on or before December 24, 2019. Addresses for mail and hand delivery of objections and hearing requests are provided in 40 CFR 178.25(b).
In addition to filing an objection or hearing request with the Hearing Clerk as described in 40 CFR part 178, please submit a copy of the filing (excluding any Confidential Business Information (CBI)) for inclusion in the public docket. Information not marked confidential pursuant to 40 CFR part 2 may be disclosed publicly by EPA without prior notice. Submit the non-CBI copy of your objection or hearing request, identified by docket ID number EPA-HQ-OPP-2018-0599, by one of the following methods:
- Federal eRulemaking Portal: http://www.regulations.gov. Follow the online instructions for submitting comments. Do not submit electronically any information you consider to be CBI or other information whose disclosure is restricted by statute.
- Mail: OPP Docket, Environmental Protection Agency Docket Center (EPA/DC), (28221T), 1200 Pennsylvania Ave. NW, Washington, DC 20460-0001.
- Hand Delivery: To make special arrangements for hand delivery or delivery of boxed information, please follow the instructions at http://www.epa.gov/?dockets/?contacts.html.
Additional instructions on commenting or visiting the docket, along with more information about dockets generally, is available at http://www.epa.gov/?dockets.
II. Summary of Petitioned-For Tolerance
In the Federal Register of June 7, 2019 (84 FR 26630) (FRL-9993-93), EPA issued a document pursuant to FFDCA section 408(d)(3), 21 U.S.C. 346a(d)(3), announcing the filing of a pesticide petition (PP 4F8338) by Dow AgroSciences LLC, 9330 Zionsville Road, Indianapolis, IN 46268. The petition requested that 40 CFR part 180 be amended by establishing tolerances for residues of sulfoxaflor (1-(6-trifluoromethylpyridin-3-yl)ethyl)(methyl)-oxido-l4-sulfanylidenecyyanamide), in or on the following raw agricultural commodities: Rice, grain at 5 parts per million (ppm); rice, straw at 5 ppm; rice, hulls at 14 ppm; and avocado, whole fruit at 0.15 ppm.
One comment was received on the notice of filing. EPA’s response to this comment is discussed in Unit IV.C.Start Printed Page 57342
Based upon review of the data supporting the petition, EPA is establishing tolerances that vary from what the petitioner requested, as authorized under FFDCA section 408(d)(4)(A)(i). EPA’s explanation for those variations are contained in Unit IV.D.
III. Aggregate Risk Assessment and Determination of Safety
Section 408(b)(2)(A)(i) of FFDCA allows EPA to establish a tolerance (the legal limit for a pesticide chemical residue in or on a food) only if EPA determines that the tolerance is “safe.” Section 408(b)(2)(A)(ii) of FFDCA defines “safe” to mean that “there is a reasonable certainty that no harm will result from aggregate exposure to the pesticide chemical residue, including all anticipated dietary exposures and all other exposures for which there is reliable information.” This includes exposure through drinking water and in residential settings but does not include occupational exposure. Section 408(b)(2)(C) of FFDCA requires EPA to give special consideration to exposure of infants and children to the pesticide chemical residue in establishing a tolerance and to “ensure that there is a reasonable certainty that no harm will result to infants and children from aggregate exposure to the pesticide chemical residue. . . .”
Consistent with FFDCA section 408(b)(2)(D), and the factors specified in FFDCA section 408(b)(2)(D), EPA has reviewed the available scientific data and other relevant information in support of this action. EPA has sufficient data to assess the hazards of and to make a determination on aggregate exposure for sulfoxaflor in or on rice and avocado.
On July 24, 2019, EPA published a final rule establishing a tolerance for residues of sulfoxaflor in or on multiple commodities based on the Agency’s conclusion that those tolerances are safe for the general population, including infants and children (84 FR 35546) (FRL-9995-63). EPA’s exposure assessment supporting the July 24, 2019 final rule included exposure from use of sulfoxaflor on both avocado and rice commodities, but these tolerances were not established at the time because a Notice of Filing for the petition for these commodities was still pending and the statutory public comment period was still open. Because the toxicity profile of sulfoxaflor has not changed since that rule was published, and because residues of sulfoxaflor on both avocado and rice were included in the dietary exposure and risk assessment supporting that rule, EPA is incorporating the findings in the preamble to that July 24, 2019 final rule into this rulemaking.
Further information about EPA’s risk assessment and determination of safety supporting the tolerances established in the July 24, 2019 Federal Register action, as well as the new sulfoxaflor tolerances on rice and avocado can be found at http://www.regulations.gov in the documents entitled: “Sulfoxaflor. Human Health Risk Assessment for New Food Uses on Numerous Crops, Ornamentals Growing in Greenhouses and Nurseries and Tree Farms and Plantations” and “Sulfoxaflor. Human Health Risk Assessment for New Food Uses on Artichoke, Asparagus, Bushberry, Caneberry and Sunflower, and Multiple Crop Group Conversions.” The documents may be found in docket ID numbers EPA-HQ-OPP-2018-0599 and EPA-HQ-OPP-2018-0179.
Therefore, based on the risk assessments and information described above, EPA concludes that there is a reasonable certainty that no harm will result to the general population, or to infants and children from aggregate exposure to sulfoxaflor residues.
IV. Other Considerations
A. Analytical Enforcement Methodology
High performance liquid chromatographic methods with positive-ion electro spray interface (ESI) and tandem mass spectrometric detection (LC/MS/MS) were previously reviewed and found to be acceptable for tolerance enforcement of sulfoxaflor residues (the two metabolites, X11719474 and X11721061, are also quantitated). The limit of quantitation (LOQ), determined as the lowest level of method validation (LLMV), is 0.010 ppm in all matrices.
The method may be requested from: Chief, Analytical Chemistry Branch, Environmental Science Center, 701 Mapes Rd., Ft. Meade, MD 20755-5350; telephone number: (410) 305-2905; email address: residuemethods@epa.gov.
B. International Residue Limits
In making its tolerance decisions, EPA seeks to harmonize U.S. tolerances with international standards whenever possible, consistent with U.S. food safety standards and agricultural practices. EPA considers the international maximum residue limits (MRLs) established by the Codex Alimentarius Commission (Codex), as required by FFDCA section 408(b)(4). The Codex Alimentarius is a joint United Nations Food and Agriculture Organization/World Health Organization food standards program, and it is recognized as an international food safety standards-setting organization in trade agreements to which the United States is a party. EPA may establish a tolerance that is different from a Codex MRL; however, FFDCA section 408(b)(4) requires that EPA explain the reasons for departing from the Codex level.
The Codex has not established a MRL for sulfoxaflor in or on avocados or rice.
C. Response to Comments
One comment was received that stated in part that tolerance levels should be zero because people do not need to be exposed to more poison. Although the Agency recognizes that some individuals believe that pesticides should be banned on agricultural crops, the existing legal framework provided by section 408 of the Federal Food, Drug and Cosmetic Act (FFDCA) authorizes EPA to establish tolerances when it determines that the tolerance is safe. Upon consideration of the validity, completeness, and reliability of the available data as well as other factors the FFDCA requires EPA to consider, EPA has determined that these sulfoxaflor tolerances are safe. The commenter has provided no information supporting a contrary conclusion.
D. Revisions to Petitioned-For Tolerance
Although proposed by the petitioner, a tolerance is not being established on rice straw because the Agency no longer considers this commodity a significant livestock feed item, per the Chemistry Science Advisory Council (ChemSAC) memorandum on livestock feed (Revisions of Feedstuffs in Table 1 of OPPTS Test Guideline 860.1000 and Guidance on Constructing Maximum Reasonably Balanced Diets (MRBD); ChemSAC; 30 June 2008). Also, based on results of the rice processing study, the Agency is establishing a tolerance of 15 ppm on rice hulls, rather than at 14 ppm as requested. This is in accordance with the pertinent rounding class of the Organization for Economic Development and Cooperation (OECD) tolerance calculation procedure.
V. Conclusion
Therefore, tolerances are established for residues of sulfoxaflor in or on avocado at 0.15 ppm; rice, grain at 5 ppm; and rice, hulls at 15 ppm.
VI. Statutory and Executive Order Reviews
This action establishes tolerances under FFDCA section 408(d) in response to a petition submitted to the Agency. The Office of Management and Budget (OMB) has exempted these types Start Printed Page 57343of actions from review under Executive Order 12866, entitled “Regulatory Planning and Review” (58 FR 51735, October 4, 1993). Because this action has been exempted from review under Executive Order 12866, this action is not subject to Executive Order 13211, entitled “Actions Concerning Regulations That Significantly Affect Energy Supply, Distribution, or Use” (66 FR 28355, May 22, 2001) or Executive Order 13045, entitled “Protection of Children from Environmental Health Risks and Safety Risks” (62 FR 19885, April 23, 1997), nor is it considered a regulatory action under Executive Order 13771, entitled “Reducing Regulations and Controlling Regulatory Costs” (82 FR 9339, February 3, 2017). This action does not contain any information collections subject to OMB approval under the Paperwork Reduction Act (PRA) (44 U.S.C. 3501 et seq.), nor does it require any special considerations under Executive Order 12898, entitled “Federal Actions to Address Environmental Justice in Minority Populations and Low-Income Populations” (59 FR 7629, February 16, 1994).
Since tolerances and exemptions that are established on the basis of a petition under FFDCA section 408(d), such as the tolerances in this final rule, do not require the issuance of a proposed rule, the requirements of the Regulatory Flexibility Act (RFA) (5 U.S.C. 601 et seq.), do not apply.
This action directly regulates growers, food processors, food handlers, and food retailers, not States or tribes, nor does this action alter the relationships or distribution of power and responsibilities established by Congress in the preemption provisions of FFDCA section 408(n)(4). As such, the Agency has determined that this action will not have a substantial direct effect on States or tribal governments, on the relationship between the national government and the States or tribal governments, or on the distribution of power and responsibilities among the various levels of government or between the Federal Government and Indian tribes. Thus, the Agency has determined that Executive Order 13132, entitled “Federalism” (64 FR 43255, August 10, 1999) and Executive Order 13175, entitled “Consultation and Coordination with Indian Tribal Governments” (65 FR 67249, November 9, 2000) do not apply to this action. In addition, this action does not impose any enforceable duty or contain any unfunded mandate as described under Title II of the Unfunded Mandates Reform Act (UMRA) (2 U.S.C. 1501 et seq.).
This action does not involve any technical standards that would require Agency consideration of voluntary consensus standards pursuant to section 12(d) of the National Technology Transfer and Advancement Act (NTTAA) (15 U.S.C. 272 note).
VII. Congressional Review Act
Pursuant to the Congressional Review Act (5 U.S.C. 801 et seq.), EPA will submit a report containing this rule and other required information to the U.S. Senate, the U.S. House of Representatives, and the Comptroller General of the United States prior to publication of the rule in the Federal Register. This action is not a “major rule” as defined by 5 U.S.C. 804(2).
List of Subjects in 40 CFR Part 180
- Environmental protection
- Administrative practice and procedure
- Agricultural commodities
- Pesticides and pests
- Reporting and recordkeeping requirements
Dated: September 30, 2019.
Michael Goodis,
Director, Registration Division, Office of Pesticide Programs.
Therefore, 40 CFR chapter I is amended as follows:
PART 180—[AMENDED]
1. The authority citation for part 180 continues to read as follows:
Authority: 21 U.S.C. 321(q), 346a and 371.
2. In §?180.668,
i. Add alphabetically entries for “Avocado”; “Rice, grain”; and “Rice, hulls” to the table in paragraph (a) to read as follows:
Sulfoxaflor; tolerances for residues.
(a) * * *
Commodity |
Parts per million |
|---|---|
Avocado |
0.15 |
Rice, grain |
5 |
Rice, hulls |
15 |
[FR Doc. 2019-23384 Filed 10-24-19; 8:45 am]
BILLING CODE 6560-50-P
*Published Document online at https://www.federalregister.gov/documents/2019/10/25/2019-23384/sulfoxaflor-pesticide-tolerances
