Key Points
- Provides an overview of the main sources of fluoride in children.
- Stresses the proportion of fluoride (F) intake from ingestion of toothpaste.
- Draws attention to the implications for oral health of the F balance in infants and young children.
- Illustrates the importance of assessing fluoride exposure at an individual and community level in the context of clinical dental practice.
Abstract
This paper provides an overview of the main sources of fluoride (F) in children and discusses the importance of assessing F exposure at an individual and community level. It describes some of the methods used to assess F exposure by estimating F intake and excretion, together with the development and use of biomarkers for F and their importance. The paper focuses on what recent F research has shown in terms of significant sources of dietary F intake in UK infants and young children and the proportion of F intake that derives from F ingestion of toothpaste. This information is considered in the context of clinical dental practice and the implications of this research for oral health discussed.
Introduction
Exposure to fluoride (F) is important for oral health although evidence now suggests that the predominant caries-preventive effects of F occur through its topical rather than systemic effects.1,2
In the UK 12% of the population benefit from the topical and systemic effects of F in tap water, while approximately 36,000 nursery and school children receive fluoridated milk on schooldays through school milk fluoridation programmes. Salt as a vehicle for F remains primarily a mainland European approach to dietary F supplementation, while prescription of F tablets is only recommended for a targeted approach to caries prevention in high caries risk children with highly motivated and compliant parental support. Consequently, we may consider that F exposure for the majority of the UK child population is primarily topical, but evidence suggests that children receive significant amounts of F through diet and toothpaste ingestion.
Following the systematic review on water fluoridation in 2000,3 the Medical Research Council’s working group4 recommended further research to measure F ingestion from all sources, as well as F excretion and retention in children and establish useful biomarkers of internal F dose for use in epidemiological studies of health effect. Although subsequent UK funding for F research to help inform policy and evidence-based practice has been limited, this paper presents evidence on child F exposure from UK-based research and discusses it in a clinical context.
For the purpose of this paper, the following databases were searched for articles on intake (or exposure) and excretion of fluoride: Ovid MEDLINE (in-process and other non-indexed citations and Ovid MEDLINE) for the period from 1946 to January 2013 and EMBASE from 1974 to January 2013. Other data sources of information were relevant publications by the World Health Organisation (WHO), UK Department of Health and the British Association for the Study of Community Dentistry (BASCD). The searches were limited to publications in English and those concerning children. Only UK-based articles reporting fluoride intake (or exposure) and sources of fluoride intake under customary dietary conditions were included. For research articles addressing both intake and excretion of fluoride in children, in view of their limited number, all globally published articles in English were included.
F intake
The common sources of ingested F include fluoridated water, foods and drinks (including infant milk formula) prepared with fluoridated water or containing F naturally. Dietary F supplements (eg fluoridated milk), non-dietary F supplements (eg F tablets), and inadvertent ingestion of fluoridated toothpastes (or other oral health products) comprise other sources of systemic F intake.
Residence in a non-water-fluoridated community does not automatically result in low F intake, nor does residence in a fluoridated community necessarily result in a higher F intake because with increasing fashion for use of bottled waters, greater consumption of soft drinks purchased and consumed away from the home and a shift towards less tap water consumption5 tap water F may not represent the F concentration of an individual’s primary fluid consumption. A study of UK bottled waters6 showed a mean F concentration of 0.08 mg/L (range 0.010.37 mg/L) and based on average water consumption in UK children7 bottled water use as a main drinking water source would reduce F intake by a mean of 38% (range 26-48%) for all ages/ both genders compared with a tap water source of 1 mg F/L. In addition, food, drink or even bottled water produced in a fluoridated area may be transported to a non-fluoridated area for consumption and vice-versa.8 This ‘halo effect’ has, with increasing globalisation, inevitably led to substantial movement of processed food and drink products across water fluoridation boundaries.
Estimation of total daily F intake is important when recommendations for F use are being considered for dental caries prevention while minimising risk of dental fluorosis. This involves being able to estimate F intake from diet and from toothpaste ingestion.
F intake from diet
Two main methods are used to estimate F intake through diet:
- A full three-day dietary history, including portion size and fluid volumes, which is collected, usually by a nutritionist, and analysed using a previously populated database containing the F concentrations of all commonly consumed foods and drinks, producing an estimated mean daily F intake for that individual
- A duplicate plate method in which an exact copy of an individual’s daily food and drink intake is collected and weighed. Solid foods are then homogenised together in a laboratory and a F concentration for the sample recorded using a F-ion selective electrode.9 A similar process is undertaken for drinks so that a total daily F intake from diet as a whole can be calculated for the individual.
When data from F intake studies of young UK children (Table 1) are considered, it can be seen that UK children aged six to seven years ingest between 0.188 mg and 0.578 mg F per day through their diet. This represents between 0.008 and 0.026 mg/kg bw/day depending on local water F concentration. Diet contributes 37% of total daily F intake (TDFI) in one-year-olds and between 43% and 65% of TDFI intake in 67-year-olds depending on drinking water F concentration. This is consistent with similar studies undertaken in other countries and confirms that solid foods contribute a significant proportion of dietary fluoride intake.10 Main dietary F sources for UK six- to seven-year-olds vary according to whether they receive a fluoridated tap water supply (Table 2). For those receiving >0.7 mgF/L water, squashes and cordials, carbonated soft drinks and tap water together account for 48% of dietary F intake while in <0.3 mgF/L areas, these drinks contribute only 22%. In contrast, for those children living in a <0.3 mgF/L, solid foods (particularly bread) provide the main dietary F source and contribute 67% of the daily dietary fluoride intake, while in a higher water F areas, rice, pasta and boiled vegetables are the main solid food contributors.
With regard to infant feeding, breastfeeding, with exclusive breastfeeding for the first four to six months, is recommended in most European countries including the UK, although for a number of reasons in many cases this may be impractical. As a result 55% of newborns are fed with infant formula and this proportion increases to 93% at four months and almost 100% at six months.11 In addition, as infants are weaned from breastfeeding they now receive the bulk of their nutrition from infant formula before moving on to cow’s milk, which is now not recommended as a substitute for breast milk or infant milk formula until the age of one year.12 For infants and very young children there is evidence that the choice of feeding method can have a profound impact on their dietary F intake. Dental fluorosis has been reported to be more prevalent in the permanent teeth of children who had been fed powdered infant milk formula diluted with the local fluoridated water supply during the first four months of life than in those who had been breast-fed.13,14 Recent studies15,16 have shown a greater impact of the F in water used to reconstitute infant powdered formula on F intake of formula-fed infants than the F content of the powdered formula itself. However, there are trends towards greater use of convenient ready-to-feed infant products and a study of UK ready-to feed infant foods17 has shown some of these to contain a relatively high F concentration (eg ready-to-feed meat-based weaning foods: 1.20 ?gF/g). More research in this area is needed since body F burden (body retention) is key to determining risk but is technically highly challenging and time-consuming in infants and young children who are not toilet-trained.
F intake from toothbrushing
Recording toothpaste ingestion is also challenging but a valid and reproducible method is available that involves measuring the amount of toothpaste dispensed onto a toothbrush and then collecting the expectorated paste and saliva produced during brushing.18 By analysing the weighed, expectorated saliva and paste for F and subtracting this value from the amount of F dispensed within the toothpaste, an estimate of inadvertent ingestion of F through toothbrushing can be made.
On average, toothbrushing with a fluoridated toothpaste accounted for 63% of TDFI in one-year-olds while for six to seven-year-olds in the UK the proportion was less, but still substantial, at between 35% and 59%, and up to 90% at an individual level (Table 1). The international literature also shows wide variations in toothpaste’s contribution to TDFI from 22% in six-year-olds in Iowa19 to 69% four to five-year-old Columbian children.20 In a recent study, conducted after the Department of Health/BASCD guidelines21 were published, F usage from fluoridated toothpaste by four to six-year-old children in a fluoridated area of the UK was investigated22 and showed that the mean weight of toothpaste dispensed was 0.67 g with no gender difference, while boys swallowed more toothpaste (0.0174 mg/kg bw/day or 44% of dispensed paste/brushing) than girls (0.0165 mg/kg bw/day; 38%). The latter study also revealed that the majority of children used, on average, more than double the Department of Health/BASCD21 recommended pea-sized amount (0.25 g). F intake per toothbrushing session was significantly influenced by the weight of toothpaste dispensed, its F concentration and the child’s age.
Total daily fluoride intake (TDFI)
Although there is currently no international consensus on the maximum safe daily dose of F, a total F intake of 0.05-0.07 mg/kg bw/day in children younger than 12 years of age is regarded as optimum for dental health benefits.23 It is also generally agreed that intakes of F should not exceed 0.1 mg/kg bw/day during infancy, to minimise the risk of dental fluorosis.23,24
As Table 1 shows, the mean TDFI for six to seven-year-olds living in UK areas where the mean water F concentration ranged from 0.08 to 1.06 mgF/L was between 0.031 and 0.076 mg/kg bw/day, while in one-year-olds living in a 0.8 mg F/L area TDFI was estimated to be 0.05 mg/kg bw/day. However, all these values represent means and the variation in TDFI at an individual level is high. This places some children potentially at risk of developing dental fluorosis if a higher F intake is sustained over a critical period of tooth development and, conversely, at the lower end of the scale provides inadequate F exposure to help prevent caries.
F excretion
Faeces, urine, and sweat represent the three different routes for the elimination of F from the body. Approximately 10% of our total daily F intake is not absorbed systemically and is consequently excreted through faeces.25,26 Urine is the most important metabolic pathway for removal of absorbed F from the body. Sweat is a quantitatively minor route of F excretion as its F concentrations are very low (1-3 ?mole/L), similar to those in plasma27 and under most conditions daily volumes are very low.
Urinary F excretion
The kidneys are the principal route for elimination of F that is absorbed systemically from the gastrointestinal tract (GIT) but not taken up by bone/other mineralised tissue, hair or nail. Under conditions of constant F intake approximately 50% of daily absorbed F becomes associated with calcified tissues within 24 hours and the rest is excreted from the body.28 However, urinary F excretion is dependent on urinary flow rate and F concentration of the urine. Urinary flow rate varies through the day depending on fluid intake, while urinary F concentration at any point in time is related to timing of F ingestion. Therefore, the best estimate of daily urinary F excretion (DUFE) is via a 24-hour urine sample and recommended by the WHO for monitoring community fluoridation schemes,29 with collection over a shorter time period if a 24-hour urine is not practical. Several F studies assessing salt fluoridation schemes have collected urine samples over 8 or 14-16 hours.30,31 In addition, although a spot (an untimed ‘single-void’) urine sample is not a valid basis for extrapolation to 24-hour data,32 the mean of two or three daily spot urine samples may provide a guide to DUFE33 and the F/creatinine ratio of a morning spot urine sample may be used to estimate mean DUFE in children who are not-toilet trained.34
Faecal F excretion
Under normal dietary intake conditions and absence of foods/drinks containing high amounts of divalent or trivalent cations such as calcium, aluminium and magnesium, which can form complexes with F in the GIT, approximately 80-90% of ingested F is absorbed from the GIT.35 Faecal F excretion in children is generally assumed to be 10% of total daily F intake. However, this assumption is based on just two studies with small numbers of very young children. A mean faecal F excretion of 11% of F intake, ranging from 2% to 15%, was reported for five breast-fed infants, whereas the corresponding mean (range) was 2% (0.5% – 5.0%) for five formula-fed infants.25 In another study Ekstrand et al.36 reported faecal F excretion from 2% to 34% of intake for formula-fed infants aged 5-11 months receiving a F supplement (0.25 mg/day) with a feed. There has been no research on faecal F excretion in UK children.
F balance, body burden, and biomarkers
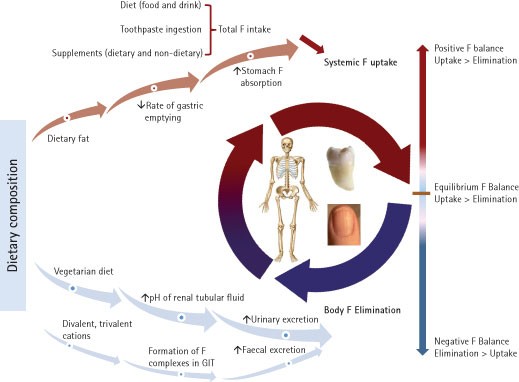
As Figure 1 illustrates, F balance represents the difference between systemic F uptake and body elimination. When total F intake is greater than total F excretion, the F balance is positive and F is retained in the body. Conversely, when F excretion is greater than F intake, the balance is negative and F is lost from the body. Several factors are known to influence the degree of F absorption and excretion, and consequently its retention and balance.
Dietary composition and the form of ingested F can affect F absorption. The absorption of sodium fluoride (NaF), a soluble F compound, added to water is almost 100%. However, the degree of absorption is reduced when F is taken with foods and drinks (such as milk) containing substantial amounts of divalent or trivalent cations due to formation of insoluble complexes that increase faecal F excretion.37 The extent of F absorption from disodium monofluorophosphate (NaMFP) and NaF in dentifrices has been suggested to be similar. However, since F in NaMFP needs to first be released by enzymatic reaction of intestinal phosphatase this can result in lower and delayed peak plasma F concentrations. In the stomach, F is mainly absorbed as hydrogen fluoride (HF) and therefore gastric F absorption is inversely associated with stomach pH. A high dietary fat intake may increase F absorption from the stomach by reducing the rate of gastric emptying.27
Although the amount of F excreted in urine is directly influenced by the amount of F intake, the daily urinary F excretion (DUFE) can be affected by several other factors. After ionic F enters the renal tubules, between 10-90% of the ion is reabsorbed and returned to the systemic circulation.38 Urinary flow rate and the pH of renal tubular fluid affect the amount of F reabsorption. With acidic tubular fluid, more ionic F is converted to HF, which is diffusible across the tubular epithelium. Conversely, with an alkaline renal tubular fluid, most F exists in ionic form and remains within the tubule to be excreted.39 Dietary composition has been shown to influence urinary pH. A meat-based diet promotes a more acidic urine, whereas a vegetarian diet renders urinary pH relatively alkaline.40 Other factors that could influence acid-base status and urinary pH include certain drugs, high altitude, some respiratory diseases, metabolic diseases such as diabetes mellitus and levels of physical activity.39
Studies on the F balance in children are limited due to the difficulty of collecting 24-hour urine and faeces samples from children and the available F balance data are conflicting. Ekstrand et al.25 found that breast-fed infants, with daily F intakes ranging from 0.005 to 0.019 mg, were in negative F balance. In contrast, they found a positive F balance for formula-fed infants who received daily F intakes ranging from 0.891 to 1.012 mg, a finding in agreement with Ericsson et al.41 who also reported a negative F balance in breast-fed infants. In another study, in three to four-year-olds residing in a fluoridated community in California F excretion in urine and faeces almost equalled F intake, and net F retention was minimal with an average F balance of +0.05 mg/day.42 Due to the practical difficulties in sampling and analysis of 24-hour faecal samples and its assumed minor role in F metabolism, most F retention and balance studies have focused their balance calculations on urinary F excretion rather than total F excretion.
Determining F body burden is important when assessing optimal F exposure for oral health benefit and conversely, dental fluorosis risk. However, collection of dietary and toothpaste ingestion data at a community level is time consuming, costly and requires a high level of expertise and therefore the development and use of biomarkers for F has been useful. This topic is extensively and comprehensively described elsewhere,43 but it is useful to summarise the widely accepted classification for biomarkers for F as it helps focus on the need for their future development as a means of improving assessment techniques for monitoring and evaluating individual- and community-based prevention. Contemporary biomarkers for F are those that assess present or very recent exposure to F; for example, blood, bone surface, saliva, milk, sweat and urine, while chronic or sub-chronic exposure to F can be assessed by recent (eg nail and hair) and historical (eg dentine and bone) biomarkers for F body burden. Urine is the current preference for estimating acute (very recent) exposure while F in nail shows some promise as a biomarker for chronic exposure and dentine in exfoliated primary teeth has been used to assess F body burden.
DUFE has been suggested as an index or biomarker for F intake. In order to clarify the ability of DUFE to predict TDFI and, therefore, the risk of development of dental fluorosis in children, a recent study examined published data on concurrent measurements of TDFI and DUFE for 212 children and suggested that children under seven years of age, on average, excrete 45% of their ingested F in the urine.44 However, studies of TDFI and DUFE have shown a wide variation in fractional urinary F excretion (FUFE), that is, the proportion of F intake that is excreted, (FUFE = [DUFE/TDFI] × 100), ranging from 32% for six to seven-year-olds to 359% for breast-fed infants as summarised in Table 3. The wide range can be explained by differences in dietary composition as well as dietary and oral hygiene habits of children studied, since these factors affect F absorption from gastrointestinal tract and urinary excretion of F, as discussed previously. Since growth rate and body size (skeletal mass) can also influence F retention, it is important to adjust the data on F intake, excretion and retention based on body weight.
Clinical relevance of F exposure in children
Chronic excessive F intake from birth to four years of age is considered to be a key contributor to dental fluorosis development in permanent maxillary central incisors.45,46 Furthermore, Levy et al.47 have suggested that the six to nine-month-old period is most important in dental fluorosis aetiology for the primary dentition, while Warren et al.48 determined that the critical period for development of fluorosis in late developing primary teeth is from four months in utero until 11 months of age.
Burt et al.49 cautioned against too precise definitions of age of greatest risk of fluorosis because there is increasing evidence that developing tooth germs may be vulnerable to F over a longer period, including their initiation period.19,50
It is important to recognise that there are a number of sources of fluorides (dietary and non-dietary) to which infants and young children may be systemically exposed during their dentally formative years. The dental impact of these sources is confounded by a number of factors relating to F metabolism and consequently F balance, described in previous sections. As the Iowa fluoride study51 has shown, the true risk factor for dental fluorosis is total F intake, which, in turn, is dependent on feeding patterns, F concentration of infant milk formula and weaning foods/drinks in infants, as well as the impact of dentifrice usage and toothbrushing habits in infants, toddlers and young children. Information on F exposure throughout the whole period of aesthetically important tooth development is critical so that contributors to dental fluorosis risk during particularly vulnerable periods can be highlighted and parents advised accordingly.
Despite the best infant feeding practice being breastfeeding, some infants may be fed exclusively on formula milk for the first six months of age. Recent guidelines from the American Dental Association in 2011 advised the parents of infants consuming reconstituted infant formula to continue using optimally fluoridated drinking water in feed preparation while being mindful of the potential risks of development of dental fluorosis.52 The guidelines also advise parents resident in a fluoridated area who are concerned about potential dental fluorosis risk to reconstitute powdered formula milk with F-free or low-F water or use ready-to-feed infant milk products. A similar approach has been adopted in the UK53 with advice to parents with concerns about dental fluorosis risk, which includes the use of suitable bottled waters for reconstituting powdered infant formula. The NHS Choices website12 provides further advice with regard to infant feeding and bottled waters stating the importance of checking the label and ensuring the sodium concentration is less than 200 mg/L and the sulphate content is not higher than 250 mg/L. It also reminds parents that bottled water is not usually sterile so that if it is being used to make up an infant feed it still needs to be boiled, like tap water, before the feed is prepared.
With regard to toothpaste use and toothbrushing, recent revisions in child toothpaste formulations and associated toothbrushing habits together with the introduction of the toolkit for prevention21 and Scottish Dental Clinical Effectiveness Programme’s guidelines for the prevention and management of decay in children54 have impacted children’s exposure to F. These evidence-based guidelines are designed to increase and maintain the topical F concentration in the mouth for a more prolonged period through the higher toothpaste F concentration and ‘spit, don’t rinse’ advice. However, to minimise the effect of F dose (dose = concentration × amount) on fluorosis risk from the inevitable inadvertent ingestion of toothpaste in children, it has become increasingly crucial that parents and children comply with manufacturers’ instructions and parents are pro-actively advised to read the instructions carefully and limit the amount of toothpaste dispensed according to the child’s age. Dispensing paste across the brush, rather than down the brush’s long axis can help with this instruction, but toothpaste manufacturers could also help by improving the dispensing system for toothpastes.
Conclusion
Although dental fluorosis is not considered an adverse health effect, but a side-effect of chronic excessive F exposure, the rational use of F requires careful monitoring since the dental fluorosis risk to caries reduction benefit ‘dose gap’ is relatively narrow. Health professionals need to be fully aware of recent and historical F histories of their young patients and families in order to assess their main sources of F exposure and other factors that may impact on body F burden. In this way they can ensure that their advice for parents and carers will be fully informed and appropriate for maximum prevention of dental caries while minimising risk of dental fluorosis in their child patients.
References
-
Fejerskov O, Thylstrup A, Larsen M J . Rational use of fluorides in caries prevention: a concept based on possible cariostatic mechanisms. Acta Odontol Scand 1981; 39: 241–249.
-
Featherstone J D . The science and practice of caries prevention. J Am Dent Assoc 2000; 131: 887–899.
-
McDonagh M, Whiting P, Bradley M et al. A systematic review of public water fluoridation (CRD report No, 18). York: NHS Centre for Reviews and Dissemination, University of York; 2000.
-
Medical Research Council. MRC Working Group report on: water fluoridation and health. London: MRC; 2002.
-
Zohouri F V, Rugg-Gunn A J, Fletcher E S et al. Changes in water intake of Northumbrian adolescents 1980 to 2000. Br Dent J 2004; 196: 547–552.
-
Zohouri F V, Maguire A, Moynihan P J . Fluoride content of still bottled waters available in the North-East of England UK. Br Dent J 2003; 195: 515–518.
-
Gregory J, Lowe S, Bates C et al. National diet and nutrition survey: young people aged 4 to 18 years. London: The Stationery Office, 2000.
-
Heilman J R, Kiritsy M C, Levy S M, Wefel J S . Assessing fluoride levels of carbonated soft drinks. J Am Dent Assoc 1999; 130: 1593–1599.
-
Martínez-Mier E A, Cury J A, Heilman J R et al. Development of gold standard ion-selective electrode-based methods for fluoride analysis Caries Res 2011; 45: 3–12.
-
Rankin S J, Levy S M, Warren J J, Gilmore J E, Broffitt B . Fluoride content of solid foods impacts daily intake. J Public Health Dent 2012; 72: 128–134.
-
Cattaneo A, Burmaz T, Arendt M et al. Protection, promotion and support of breast-feeding in Europe: progress from 2002 to 2007. Public Health Nutr 2010; 13: 751–759.
-
NHS Choices. Pregnancy and baby: what milk when? NHS, 2013. Online information available at http://www.nhs.uk/conditions/pregnancyandbaby/pages/solidfoods weaning.aspx (accessed May 2013).
-
Walton J L, Messer L B . Dental caries and fluorosis in breast-fed and bottled-fed children. Caries Res 1981; 15: 124–137.
-
Fomon S J, Ekstrand J . Fluoride intake by infants. J Public Health Dent 1999; 59: 229–234.
-
Cressey P . Dietary fluoride intake for fully formula fed infants in New Zealand: impact of formula and water fluoride. J Public Health Dent 2010; 70: 285–291.
-
Zohoori F V, Moynihan P J, Omid N, Abuhaloob L, Maguire A . Impact of water fluoride concentration on the fluoride content of infant foods and drinks requiring preparation with liquids before feeding. Community Dent Oral Epidemiol 2012; 40: 432–440.
-
Maguire A, Omid N, Abuhaloob L, Moynihan P J, Zohoori F V . Fluoride content of Ready-toFeed (RTF) infant food and drinks in the UK. Community Dent Oral Epidemiol 2012; 40: 26–36.
-
Maguire A, Zohouri F V, Hindmarch P N, Hatts J, Moynihan P J . Fluoride intake and urinary excretion in 6to 7yearold children living in optimally, sub-optimally and non-fluoridated areas. Community Dent Oral Epidemiol 2007; 35: 479–488.
-
Levy S M, Warren J J, Broffitt B . Patterns of fluoride intake from 36 to 72 months of age. J Public Health Dent 2003; 63: 211–220.
-
Franco A M, Martignon S, Saldarriaga A et al. Total fluoride intake in children aged 22–35 months in four Colombian cities. Community Dent Oral Epidemiol 2005; 33: 1–8.
-
Department of Health and British Association for the Study of Community Dentistry. Delivering better oral health; an evidence-based toolkit for prevention. London: DH, 2007.
-
Zohoori F V, Duckworth R M, Omid N, O’Hare W T, Maguire A . Fluoridated toothpaste: usage and ingestion of fluoride by 4-to 6-yr-old children in England. Eur J Oral Sci 2012; 120: 415–421.
-
Institute of Medicine. Dietary reference intakes for calcium, magnesium, vitamin D and fluoride. Washington DC: National Academy Press, 1999.
-
European Food Safety Authority (EFSA). Opinion of the scientific panel on dietetic products, nutrition and allergies on a request from the commission related to the tolerable upper intake level of fluoride. The EFSA Journal 2005; 192: 1–65.
-
Ekstrand J, Hardell L I, Spak C J . Fluoride balance studies on infants in a 1ppmwater-fluoride area. Caries Res 1984; 18: 87–92.
-
Ekstrand J, Ziegler E E, Nelson S E, Fomon S J . Absorption and retention of dietary and supplemental fluoride by infants. Adv Dent Res 1994; 8: 175–180.
-
Whitford G M . The metabolism and toxicity of fluoride. 2nd edn. Basel: Karger, 1996.
-
Whitford G M . Intake and metabolism of fluoride. Adv Dent Res 1994; 8: 5–14.
-
Marthaler T M . Monitoring of renal fluoride excretion in community preventive programmes on oral health. Geneva: World Health Organization, 1999.
-
Marthaler T M, Steiner M, Menghini G, De Crousaz P . Urinary fluoride excretion in children with low fluoride intake or consuming fluoridated salt. Caries Res 1995; 29: 26–34.
-
Marthaler T M . Salt fluoridation in Europe, comparisons with Latin America. Amsterdam: Proceedings of the 8th World Salt Symposium, 2000; 2: 1021–1025.
-
Warpeha R A, Marthaler T M . Urinary fluoride excretion in Jamaica in relation to fluoridated salt. Caries Res 1995; 29: 35–41.
-
Bean N E, Rugg-Gunn A, Wright W G, Eastoe J E . Fluoride concentrations in limited urine samples from human volunteers compared with their daily fluoride output. Caries Res 1989; 23(103): Abstr 44.
-
Zohouri F V, Swinbank C M, Maguire A, Moynihan P J . Is the fluoride/creatinine ratio of a spot urine sample indicative of 24h urinary fluoride? Community Dent Oral Epidemiol 2006; 34: 130–138.
-
Cremer H D, Büttner W . Absorption of fluorides. Monogr Ser World Health Organ 1970; 59: 75–91.
-
Ekstrand J, Fomon S J, Ziegler E E, Nelson S E . Fluoride pharmacokinetics in infancy. Pediatr Res 1994; 35: 157–163.
-
Whitford G M . Effects of plasma fluoride and dietary calcium concentrations on GI absorption and secretion of fluoride in the rat. Calcif Tissue Int 1994; 54: 421–425.
-
Ekstrand J . Fluoride metabolism. In Fejerskov O, Ekstrand J, Burt B A (eds) Fluoride in dentistry. pp 55–68. Copenhagen: Munksgaard, 1996.
-
Whitford G M . The physiological and toxicological characteristics of fluoride. J Dent Res 1990; 69: 539–549.
-
Ekstrand J, Spak C J, Ehrnebo M . Renal clearance of fluoride in a steady state condition in man: influence of urinary flow and pH changes by diet. Acta Pharmacol Toxicol 1982; 50: 321–325.
-
Ericsson Y, Hellstrom I, Hofvander Y . Pilot studies on the fluoride metabolism in infants on different feedings. Acta Paediatr Scand 1972; 61: 459–464.
-
Brunetti A, Newbrun E . Fluoride balance of children 3 and 4 years old. Caries Res 1983; 17: 171 (abstract 41).
-
Buzalaf M A R . Fluoride and the oral environment. Basel: Karger, 2011.
-
Villa A, Anabalon M, Zohouri V, Maguire A, Franco A M, Rugg-Gunn A . Relationships between fluoride intake, urinary fluoride excretion and fluoride retention in children and adults: an analysis of available data. Caries Res 2010; 44: 60–68.
-
Evans R W, Darvell B W . Refining the estimate of the critical period for susceptibility to enamel fluorosis in human maxillary central incisors. J Public Health Dent 1995; 55: 238–249.
-
Bardsen A . ‘Risk periods’ associated with the development of dental fluorosis in maxillary permanent central incisors: a meta-analysis. Acta Odontol Scand 1999; 57: 247–256.
-
Levy S M, Hillis S L, Warren J J et al. Primary tooth fluorosis and fluoride intake during the first year of life. Community Dent Oral Epidemiol 2002; 30: 286–295.
-
Warren J J, Levy S M, Kanellis M J . Prevalence of dental fluorosis in primary dentition. J Public Health Dent 2001; 61: 87–91.
-
Burt B A, Keels M A, Heller K E . The effects of a break in water fluoridation on the development of dental caries and fluorosis. J Dent Res 2000; 79: 761–769.
-
Robinson C, Kirkham J, Weatherell J A . Fluoride in teeth and bone. In: Fejerskov O, Ekstrand J, Burt B A (eds) Fluoride in dentistry. Copenhagen: Munksgaard 1996.
-
Levy S M, Broffitt B, Marshall T A, Eichenberger-Gilmore J M, Warren J J . Associations between fluorosis of permanent incisors and fluoride intake from infant formula, other dietary sources and dentifrice during early childhood. J Am Dent Assoc 2010; 141: 1190–1201.
-
Berg J, Gerweck C, Hujoel P P et al. Evidence-based clinical recommendations regarding fluoride intake from reconstituted infant formula and enamel fluorosis: a report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011; 142: 79–87.
-
British Fluoridation Society. Water fluoridation and infant formula a British Fluoridation Society briefing paper. British Fluoridation Society, 2008. Online article available at http://www.bfsweb.org/InfantF revised Jul08 final.htm (accessed May 2013).
-
Scottish Dental Clinical Effectiveness Programme (SDCEP). Prevention and management of dental caries in children. Dental Clinical Guidance. Dundee: SDCEP 2010.
-
Villa A, Anabalon M, Cabezas L . The fractional urinary fluoride excretion in young children under stable fluoride intake conditions. Community Dent Oral Epidemiol 2000; 28: 344–355.
-
Franco A M, Saldarriaga A, Martignon S et al. Fluoride intake and fractional urinary fluoride excretion of Colombian preschool children. Community Dent Health 2005; 22: 272–278.
-
Haftenberger M, Viergutz G, Neumeister V, Hetzer G . Total fluoride intake and urinary excretion in German children aged 3–6 years. Caries Res 2001; 35: 451–457.
-
Zohouri F V, Rugg-Gunn A J . Total fluoride intake and urinary excretion in 4yearold Iranian children residing in low-fluoride areas. Br J Nutr 2000; 83: 15–25.
-
Zohoori F V, Walls R, Teasdale L et al. Fractional urinary fluoride excretion of 6–7year-old children attending schools in low-fluoride and naturally fluoridated areas in the UK. Br J Nutr 2012; 14: 1–7.
-
Zohoori F V, Maguire A, Moynihan P J . Sources of dietary fluoride intake in 6–7 year old English children receiving optimally, sub-optimally, and non-fluoridated water. J Public Health Dent 2006; 66: 227–234.
Additional information
Refereed paper
Rights and permissions
About this article
Cite this article
Maguire, A., Zohoori, F. Fluoride balance in infants and young children in the UK and its clinical relevance for the dental team. Br Dent J 214, 587–593 (2013). https://doi.org/10.1038/sj.bdj.2013.531
- Accepted
- Published
- Issue Date
- DOIhttps://doi.org/10.1038/sj.bdj.2013.531
Further reading
-
Brick tea consumption is a risk factor for dental caries and dental fluorosis among 12-year-old Tibetan children in Ganzi
Environmental Geochemistry and Health (2019)
-
Fluoride levels in UK infant milks
European Archives of Paediatric Dentistry (2016)