|
ACTIVITY:
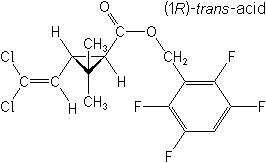
Insecticide (pyrethroid)
CAS Name:
(2,3,5,6-tetrafluorophenyl)methyl (1R-trans)-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
Structure:
Reports
available from
The National Technical Information Service
(NTIS)
Order from NTIS by: phone at 1-800-553-NTIS (U.S. customers);
(703)605-6000 (other countries); fax at (703)605-6900; and
email at orders@ntis.gov. NTIS is located at 5285 Port Royal
Road, Springfield, VA, 22161, USA. |
| Order
No. |
Title |
Keywords |
CAS
Nos. |
NTIS/OTS0543290-2
EPA/OTS;
Doc #89970000165 |
1997
- SUPPORT: NAK 4455 - STUDY FOR CHRONIC TOXICITY AND CANCEROGENICITY
IN WISTAR RATS (ADMINISTRATION IN DIET FOR 2 YEARS), WITH
TSCA HLTH & SFTY STUDY CVR SHT DATED 5/8/97
BAYER
AG |
BAYER
CORP
(2,3,5,6-TETRAFLUOROPHENYL)-
METHYL-(1R-TRANS)-3-(2,2-
DICHLO*
HEALTH EFFECTS
CHRONIC TOXICITY
COMBINED CHRONIC TOXICITY/CARCINOGENICITY
MAMMALS
RATS
ORAL
DIET |
118712-89-3
|
NTIS/OTS0543287-1
EPA/OTS;
Doc #89-950000175 |
1995
-
SUPPORT: ASSESSMENT OF RESULTS OF SPECIFIC MOUSE AND RAT
CHRONIC TOXICITY/ONCOGENICITY STUDIES OF NAK 4455, WITH
COVER LETTER DATED 03/31/95
JELLINEK,
SCHWARTZ & CONNOLLY
|
MILES
INC
NAK 4455
HEALTH EFFECTS
CHRONIC TOXICITY
COMBINED CHRONIC TOXICITY/CARCINOGENICITY
MAMMALS
RATS
ORAL
DIET
CARCINOGENICITY
MICE |
118712-89-3 |
NTIS/OTS0543290-1
EPA/OTS;
Doc #89-950000174 |
1995
-
SUPPORT: ASSESSMENT OF COMBINED CHRONIC TOXICITY/ONCOGENICITY
RAT AND MOUSE STUDIES OF NAK 4455, WITH COVER LETTER DATED
03/31/95
JELLINEK,
SCHWARTZ & CONNOLLY
|
MILES
INC
NAK 4455
HEALTH EFFECTS
CHRONIC TOXICITY
COMBINED CHRONIC TOXICITY/CARCINOGENICITY
MAMMALS
RATS
ORAL
DIET
CARCINOGENICITY
MICE |
118712-89-3
|
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15921213&query_hl=2
Am
J Rhinol. 2005 Mar-Apr;19(2):141-51.
Genotoxic effects of pentachlorophenol, lindane,
transfluthrin, cyfluthrin, and natural pyrethrum on
human mucosal cells of the inferior and middle nasal
conchae.
Tisch
M, Faulde MK, Maier H.
Department
of Otorhinolaryngology, Head and Neck Surgery, Bundeswehr
Hospital, Ulm, Germany.
BACKGROUND:
Animal experiments and epidemiological
studies suggest that pentachlorophenol (PCP)
and gamma-hexachlorocyclohexane (lindane) should be
classified as possible human carcinogens. In the past,
both have had a variety of applications in the civilian
and military sectors and in forestry. They have, e.g.,
been used to impregnate and treat uniforms and other
fabrics and to control human lice. Animal experiments
indicate that PCP in particular causes mutations and
chromosome aberrations and thus DNA damage. Studies
on whether or not this also applies to newer substances
and especially to natural type I and type II pyrethroids
still are not available. What is particularly lacking
are data on the genotoxic effects of these substances
on human target cells. Our study
describes the genotoxic effects of PCP, lindane,
transfluthrin, cyfluthrin,
and natural pyrethrum on human
mucosal cells of the inferior and middle nasal conchae.
METHODS: Epithelial cells were isolated from nasal mucosa,
which was removed in the surgical treatment of chronic
sinusitis and nasal concha hyperplasia. After the cells
had been tested for vitality using the trypan blue exclusion
test, the short-term culture method was used. The material
was incubated with PCP (0.3, 0.75, and 1.2 mmol), lindane
(0.5, 0.75, and 1.0 mmol), transfluthrin (0.05, 0.1,
0.5, 0.75, and 1.0 mmol), cyfluthrin (0.05, 0.1, 0.5,
0.75, and 1.0 mmol), natural pyrethrum (0.001, 0.005,
0.01, 0.05, and 0.1 mmol), and N-methyl-N'-nitro-N-nitrosoguanidine
for 60 minutes. Substance-induced DNA damage (single-strand
and double-strand breaks) were determined using single-cell
microgel electrophoresis. A fluorescence microscope
was used together with an image processing system to
analyze the results obtained.
RESULTS: After exposure to all tested substances, a
high percentage of the cells of the middle nasal concha
in particular were found to have severely fragmented
DNA as a result of strong genotoxic effects. Although
the reaction of the cells of the inferior nasal concha
was significantly less strong (p < 0.001), the tested
substances were nevertheless found to have a notable
genotoxic effect on these cells too.
CONCLUSION: Our study strongly
suggests that exposure to PCP, lindane, transfluthrin,
cyfluthrin, and natural pyrethrum has a genotoxic effect
on the epithelial cells of human nasal mucosa.
In addition, we have shown that nasal structures differ
in susceptibility to the various pesticides used in
the tests. Thus, the study provides new evidence supporting
the biological plausibility of PCP- and lindane-induced
effects, thereby helping evaluate potential PCP- and
lindane-induced mucous membrane carcinomas of these
parts of the nose. In addition, our study shows that
other substances that today are widely used for controlling
pests have a considerable genotoxic effect on human
target cells. The results obtained indicate the need
for additional studies on the genotoxicity of these
substances and their adverse effects on human health.
PMID:
15921213 [PubMed - in process]
|
From Toxline
at Toxnet
PESTICIDE SCIENCE; 52 (1).
1998. 3-20.
Research
into fluorinated pyrethroid alcohols: An episode in
the history of pyrethroid discovery.
NAUMANN K
Landwirtschaftszentrum Monheim,
Bayer AG, D-51368 Leverkusen, Germany.
BIOSIS COPYRIGHT: BIOL ABS. An account of pyrethroid
research from 1975 to 1985 at Bayer AG is given. The
exploitation of fluorine chemistry for this purpose
led to increased activity of known 3-phenoxybenzyl pyrethroid
esters and to the commercialization of the broad-spectrum
insecticide cyfluthrin, the particularly tick-toxic
flumethrin and the rapid-acting household insecticides
fenfluthrin and transfluthrin.
The last two constituted in 1976 a novel type of pyrethroid,
based on polyfluorinated benzyl alcohols, off the mainstream
of published pyrethroid research. Transfluthrin, the
single isomer (1R)trans-permethric acid ester of 2,3,5,6-tetrafluorobenzyl
alcohol has just been introduced to the market. The
history of its discovery and structure-activity data
as well as resistance considerations regarding cyfluthrin,
are presented.
|
Report:
Evaluation
on: Transfluthrin Use as a Public Hygiene Insecticide.
September 1997.
Prepared
by: the UK Health and Safety Executive, Biocides &
Pesticides Assessment Unit, Magdalen House, Stanley
Precinct, Bootle, Merseyside L20 3QZ. Available from:
Department for Environment, Food and Rural Affairs,
Pesticides Safety Directorate, Mallard House, Kings
Pool, 3 Peasholme Green, York YO1 7PX. UK.
Available at:
http://www.pesticides.gov.uk/citizen/evaluations/165_confirm-box.htm
|
•
Note from FAN: The following study
seems promising (albeit that we are expected to be the
unwitting repositories of industrial chemicals and pesticides).
Of interest, transfluthrin is not registered for use
in the US, yet it has been included in this study supported
by US agencies. Also, according to the UK report, cited
above, animal studies showed high levels of fluoride
in the teeth and bone. - EC.
From Toxline at Toxnet
Fetal
exposure to environmental toxins
& infant outcome
Year
of Publication: 2002
Authors:
OSTREA EMJR
Author
Address: EOSTREA@MED.WAYNE.EDU, HUTZEL HOSPITAL, 4707
ST ANTOINE BOULEVARD, DETROIT, MI 48201
Source:
Crisp Data Base National Institutes of Health
Supporting
Agency: U.S. DEPT. OF HEALTH AND HUMAN SERVICES; PUBLIC
HEALTH SERVICE; NATIONAL INSTITUTES OF HEALTH, NATIONAL
INSTITUTE OF CHILD HEALTH AND HUMAN DEVELOPMENT
DESCRIPTION
(provided by applicant): The exposure of pregnant women
to environmental toxins is of major concern because
of their potential harm on the fetus. However, the detection
of fetal exposure to environmental toxins still remains
a major challenge. We propose that meconium analysis
is a promising tool to meet this challenge.
Aims:
(1) To compare the prevalence
and amount of fetal exposure to environmental toxins
through the analysis of meconium, cord blood and neonatal
hair and to determine the degree of agreement among
these three methods,
(2) to determine the relationship between the prevalence
and amount of maternal exposure to environmental toxins
during pregnancy, as determined by serial analyses of
maternal hair and blood, to the prevalence and amount
of fetal exposure to environmental toxins as determined
by meconium, cord blood and neonatal hair analyses,
and
(3) to compare adverse immediate (birth weight, length,
head circumference, gestational age) and long term (postnatal
growth and neurobehavioral development up to 2 yrs from
enrollment) outcomes that are associated with antenatal
exposure to environmental toxins as determined by maternal
blood, maternal hair, meconium, cord blood and neonatal
hair analyses.
Study design: Pregnant
women (n=750) will be recruited, at midgestation, from
the Outpatient Clinic of the Bulacan Provincial Hospital,
Philippines and their blood and hair will be
obtained at the time of recruitment and at delivery.
Umbilical cord blood, meconium and neonatal hair will
also be obtained. The samples will be analyzed, by atomic
absorption spectrometry, for lead, mercury and cadmium
and by gas chromatography/mass spectrometry for the
following pesticides and their metabolites: propoxur,
transfluthrin, Malathion,
DDT, chlorpyrifos, bioallethrin, pretilachlor, lindane,
cyfluthrin and cypermethrin. Pertinent maternal and
infant data will be obtained after birth. The infants
will be subsequently followed up at scheduled intervals
for 2 years, to study their physical growth and neurobehavioral
development using a battery of tests.
Data analysis: The relationship
between the presence/amount of environmental toxins
in meconium, maternal blood, maternal hair, cord blood
or neonatal hair to the immediate and two year outcome
in the infants will be studied, while controlling for
potential confounders. The presence/amount of environmental
toxins in maternal blood, hair, cord blood, meconium
and neonatal hair will be also evaluated to determine
which substrate (s) provide(s) the best index of exposure
for a given toxin.
Expected benefits: Meconium
analysis may provide a powerful tool to study the prevalence
and degree of fetal exposure to environmental toxins
and its associated adverse effects. This project can
also serve as a model for the study of environmental
pollutant problems during pregnancy at a local, national
or global level.
|
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15018801
J Chromatogr B Analyt Technol Biomed Life Sci. 2004
Apr 5;802(2):371-6.
Electron ionization gas chromatography-mass
spectrometric determination of residues of thirteen pyrethroid
insecticides in whole blood.
Ramesh A, Ravi PE.
Department of Analytical Chemistry, Mass Spectrometry Division,
International Institute of Biotechnology and Toxicology-IIBAT,
Padappai, Chennai 601301, Tamil Nadu, India. raamesh_a@hotmail.com
A new rapid and sensitive electron ionization gas chromatography-mass
spectrometry method in selective ion monitoring mode (SIM) was
developed for the determination of l3 synthetic pyrethroid insecticide
molecules and their stereo isomers in whole blood. The pyrethroid
insecticides investigated are allethrin, bifenthrin, cypermethrin,
cyphonothrin, cyfluthrin, lambda-cyhalothrin, deltamethrin,
fenvalerate, fenpropathrin, imiprothrin, permethrin, prallethrin
and transfluthrin. The residues
of pyrethroids are extracted from the whole blood using hexane
and acetone mixture (80 + 20%) as solvent. All the pyrethroid
residues were separated by using a gas chromatography-mass spectrometry
operated in electron ionization mode and quantified in selective
ion monitoring mode. The method can detect the residues of different
pyrethroids down to the level 0.05-2 ng/ml. Recovery experiments
conducted in whole blood samples at the fortification level
1-1000 ng/ml showed 91-103% recovery. The applications of the
analytical method for the determination of pyrethroid residues
in real samples were tested by analyzing 45 human blood samples
collected from the population exposed continuously to different
pyrethroid based formulations. The results are confirmed by
spiking the known quantity of pyrethroids and subsequently their
positive detection.
PMID: 15018801 [PubMed - indexed for MEDLINE]
http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12243228&dopt=Abstract
Med Vet Entomol. 2002 Sep;16(3):277-84.
Personal protection against mosquitoes
in Dar es Salaam, Tanzania, by
using a kerosene oil lamp to vaporize transfluthrin.
Pate HV, Line JD, Keto AJ, Miller JE.
Department of Infectious and Tropical Diseases, London School
of Hygiene and Tropical Medicine, UK. helen.pates@lshtm.ac.uk
The effectiveness of a cheap and easy method of household protection
against Culex quinquefasciatus Say and other mosquitoes (Diptera:
Culicidae) was investigated in Dar es Salaam, Tanzania. Kerosene-burning
lamps (korobois) were modified to heat and vaporize transfluthrin,
a volatile pyrethroid insecticide. When
transfluthrin was added to fuel of the lamp, protection against
biting was poor unless a very high concentration of insecticide
was used. A modified lamp (= vaporizing koroboi) was
designed to overcome this problem by mixing the insecticide
with vegetable oil and heating it to 120 degrees C in a tin
held just above the flame. The concentration of 0.1% transfluthrin
in vegetable oil gave 50-75% reduction in biting, a similar
degree of protection to that obtained from burning a mosquito
coil containing a synthetic pyrethroid (0.25% d-allethrin) and
significantly better protection than a locally bought coil (brand
'White Crane', probably containing DDT). Greater
protection (consistently > 90%) was achieved with a higher
concentration of transfluthrin (0.5%) in the vegetable oil.
This modified lamp is simple, cheap and employs locally
available technology. With further development, and
due regard to inhalation toxicity of the vaporized materials,
it may offer a more cost-effective alternative to a mosquito
coil as a means of personal protection, and a useful complement
to a net for the early part of the evening before bedtime.
Publication Types:
• Clinical Trial
PMID: 12243228 [PubMed - indexed for MEDLINE]
http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11354726&dopt=Abstract
J Environ
Monit 2001 Feb;3(2):191-3
Monitoring
of allethrin, deltamethrin, esbiothrin, prallethrin and transfluthrin
in air during the use of household
mosquito repellents.
Ramesh A, Vijayalakshmi A.
Department of Pesticide Chemistry, Fredrick
Institute of Plant Protection and Toxicology, Padappai-601 301,
Kancheepuram District, Tamil Nadu, India. raamesh_a@hotmail.com
Three types of mosquito repellent [two different
mosquito coils containing allethrin 0.1% w/w and transfluthrin
0.03% w/w, an aerosol sample containing a combination of two
pyrethroid molecules (deltamethrin 0.02% w/w + allethrin 0.13%
w/w) and two different mosquito mats containing esbiothrin 2.0%
w/w and prallethrin 1.5% w/w as active ingredients] were individually
subjected to use in a closed room. Air samples from the room
were drawn at different time intervals (15, 30 and 45 min, and
1, 2, 4, 6 and 8 h) uniformly from three different positions
in the room (top, middle and bottom) with the pyrethroid contents
analysed using gas chromatography-electron capture detection
(GC-ECD). Analysis of air samples showed maximum concentrations
of the pyrethroid residues allethrin (0.0120 ppm),
transfluthrin (0.0134 ppm), deltamethrin (0.0057 ppm),
allethrin (0.080 ppm), esbiothrin (0.015 ppm) and prallethrin
(0.0138 ppm) within 30-45 min of use. The drop in residue content
was significant with time. At the end of a 6 h period, most
of the residues had dissipated to below 0.0001 ppm. Further
significant differences were observed in the residue contents
tested at different points within the room. Studies were compared
with the experimental results obtained when the mosquito repellents
were tested with air circulation in the room.
PMID: 11354726 [PubMed - indexed for MEDLINE]
Return
to Transfluthrin Index page
|