The Fluoride Action Network (FAN) has obtained data showing that the risk of fluoride to bone and joints is far worse than U.S. health authorities have thus far acknowledged. The data, which have never before been considered by any government, scientific, or public health organization in the U.S., show that current safety standards do not protect against fluoride-induced joint pathologies, including osteoarthritis, and many people whose bones and joints are seriously damaged by fluoride exposure will not be identified as suffering from fluoride toxicity under prevailing diagnostic standards endorsed by U.S. health authorities. Based on the previously unconsidered data, a serious reassessment about the safety of current fluoride policies is urgently needed.
Current “Safe” Fluoride Dose Is Toxic to Bone
In the United States, the Institute of Medicine (IOM) has established 10 milligrams as the dose of fluoride that anyone over age 8 can ingest every day of their life without experiencing any toxic effect on their bone — including those with kidney disease. According to the IOM, it’s only when the dose exceeds 10 mg/day that fluoride can cause the earliest stages of skeletal fluorosis (a bone disease caused by fluoride). The IOM’s safety standard has long been known to be riddled with problems; today, FAN can state unequivocally that it is — empirically — false.
FAN has obtained and translated the findings of a comprehensive study from China which shows that skeletal fluorosis becomes detectable by x-ray (“stage 1” fluorosis) at daily doses of just 6.5 mg/day (Expert Group, 2000). The study, which was the result of a coordinated research effort by a team of Chinese health agencies is the only study of its kind to systematically investigate the fluoride dose that causes the various stages of skeletal fluorosis. To do so, the health agencies focused on populations in China that receive fluoride almost exclusively from a tea drink known as “brick tea.” Based on their findings, the health agencies concluded that “the daily intake of fluoride from brick tea must not exceed 5 mg.” To put this in context, the U.S. Department of Health and Human Services has estimated that adults in fluoridated communities ingest up to 6.6 mg/day (DHHS 1991).
The health agencies’ findings are consistent with the findings of other research teams in China. In 2003, for example, a study published in the western journal Food & Chemical Toxicology documented the development of crippling skeletal fluorosis (“stage 3” fluorosis) at daily doses of just 9 to 12 mg/day. Obviously, if crippling fluorosis can occur at 9 to 12 mg/day, far lower doses can cause the earlier stages of the disease. As noted by the authors:
“The present paper shows that daily intakes of 9-12 mg are associated with a very high prevalence of skeletal fluorosis. [The US Institute of Medicine’s] upper safe limit may need to be revised/lowered on the basis of present data.” (Cao 2003)
Skeletal Fluorosis Occurs at “Safe” and “Optimal” Fluoride Levels in Water
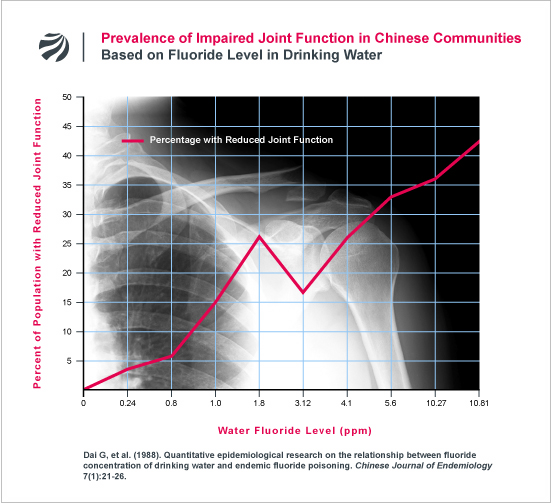
In addition to the above, FAN has obtained data that contradicts a key premise upon which current drinking water standards for fluoride are based. In 1985, the U.S. Environmental Protection Agency (EPA) established the maximum allowable level of fluoride in U.S. water supplies at 4 ppm. The EPA established this level based, in part, on the Agency’s conclusion that skeletal fluorosis does not occur anywhere in the world, including countries like India and China, where water contains less than 4 ppm fluoride (EPA Criteria Document, 1985, p. 48). In sharp contrast to EPA’s assertion, FAN has obtained data showing that skeletal fluorosis consistently occurs in Chinese communities at fluoride levels not just below 4 ppm, but below 1 ppm — the level purposely added to U.S. water supplies to prevent tooth decay. (Huang 2009; Dai 1988). In Dai (1988), skeletal fluorosis was found in communities with 0.8 and 1 ppm, while in Huang (2009), skeletal fluorosis was found in a communities with just 0.5 ppm and 1 ppm. Based on these findings, Dai and colleagues expressly warned that “we should not view areas in the 0.8-1.0 mg/L fluoride concentration range as safe.” (Dai 1988). For more research on this issue, click here.
Fluoride Causes Osteoarthritis
It has long been known that skeletal fluorosis can cause degenerative changes in bone tissue and cartilage, as well as symptoms of joint pain and stiffness, that both closely resemble osteoarthritis. In the United States, osteoarthritis affects over 27 million people and is the number one cause of disability. Despite this, there has, until now, been a striking absence of research on the relationship between osteoarthritis and fluoride exposure. This paucity of research was starkly highlighted in 2006, when the National Research Council (NRC) observed that (1) “There are no data from which a dose-response relationship can be drawn regarding fluoride intake and arthritis in humans” and, (2), only one study has “attempt[ed] to link fluoride exposure to the induction of arthritis (osteoarthritis).” (NRC 2006, p. 178). Based on the data that FAN has recently obtained, it is now clear that far more research has been done on this subject than previously recognized by the public health community and the NRC. This research shows that fluoride exposure can directly cause overt osteoarthritis.
The ability of fluoride to cause osteoarthritis was convincingly demonstrated in a large study by a Chinese research group (Bao 2003). In the study, the researchers x-rayed the right hands of adults living in fluorosis areas of China; these x-rays were then compared with the x-rays of nearby non-fluorosis areas as well as the x-rays of a nationwide study that the authors had previously conducted. When crunching the numbers, the researchers found that the incidence of osteoarthritis in the fluorosis areas were “remarkably higher” than either the adjacent areas or the nation as a whole. Whereas the osteoarthritis rate was 23.6% in the adjacent non-fluorosis areas and 24.4% for the nation as a whole, the osteoarthritis rate in the fluorosis areas was a remarkable 59%.
Not only was the rate of osteoarthritis in the fluorosis area dramatically elevated, the researchers noted that they could find no tell-tale distinctions in the osteoarthritis among the fluorosis patients that would differentiate it from normal osteoarthritis. As noted by the researchers: “the osteoarthritis caused by fluorosis differs from ordinary osteoarthritis in severity rather than in nature.”
This study is but one of several that FAN has obtained that demonstrate a link between fluoride exposure and osteoarthritis. Other studies obtained by FAN include a meticulously detailed report on the osteoarthritic effects in the elbows of adults suffering from skeletal fluorosis (Chen 1988), studies on fluoride-induced osteoarthritis in the knee and hip (Luo 2012; Su 2012), and a large study of Chinese populations, involving more than 7,000 individuals, which found that the symptoms of osteoarthritis greatly increase as the fluoride level in a community’s drinking water supply increases (Ge 2006). The results of the latter study are displayed in the following figure.
As can be seen in the figure, the incidence of joint pain and rigidity were notably elevated at just 1.7 ppm fluoride in water (a level currently deemed “safe” in the U.S.). In the community with the lowest level of fluoride in water (0.3 ppm), the incidence of knee and spinal rigidity were 3% and 7%, respectively. By contrast, in the community with 1.7 ppm, the rates were 17% and 21% — three to five times greater than the low-fluoride community. Similar findings can be seen in the rates for joint pain as well.
An earlier study from China found a similar deterioration in joint mobility (Dai 1988). The following figure plots the data from this earlier study:
Fluoride Causes Arthritis BEFORE Skeletal Fluorosis Is Detectable
Finally, FAN has obtained data that further undermines the single most important premise underlying current fluoride safety standards. For decades, U.S. health authorities have insisted that the joint pathologies resulting from fluoride exposure only occur among people with advanced stages of skeletal fluorosis. Under this longstanding doctrine, if a person does not have x-ray evidence of osteosclerosis in their spine, any symptoms of joint pain and stiffness are deemed unrelated to fluoride exposure.
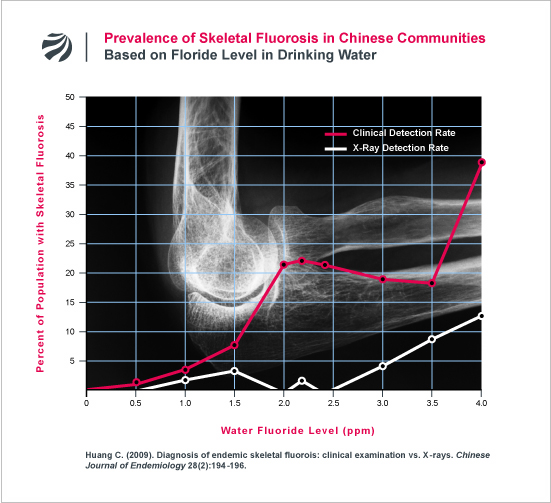
The erroneous nature of this doctrine is highlighted by the heretofore unknown findings of a Chinese research team led by Changqing Huang (arguably China’s most pre-eminent scientist on skeletal fluorosis). In 2009, Huang’s research team reported the results of an intensive investigation which found that a large percentage of individuals who are diagnosed with skeletal fluorosis based on their clinical symptoms (e.g., reduced joint mobility, joint pain, etc) do not have fluorotic bone changes that are detectable on x-ray (Huang 2009). Huang’s study found that the number of people with clinical symptoms of fluorosis markedly increases as the fluoride level in water increases and greatly outnumbers those with fluorosis that can be detected by x-ray. Indeed, as can be seen in the following figure, over 20% of the people in a community can suffer clinical symptoms of fluorosis without anyone in the community suffering a form of fluorosis that is detectable by x-ray examination.
Huang’s findings, which are consistent with a long line of other research that FAN has extensively documented here and here, demonstrate that the longstanding doctrine that fluorosis does not impact a person’s health until x-ray fluorosis is readily apparent is deeply misguided.
The practical implications of Huang’s finding are manifold. First, any safety standard for fluoride that only protects against x-ray fluorosis will not protect against fluoride-induced joint pathologies. Second, since skeletal fluorosis with x-ray detectable bone changes has been observed at daily doses of 6.5 mg/day, the pre-skeletal symptoms of fluorosis (e.g., joint pain/stiffness) can likely occur at daily doses below 6.5 mg/day — a dose that some adults in fluoridated communities in the U.S. are estimated to receive (DHHS 1991). And, third, doctors who require x-ray evidence of fluorosis before making a diagnosis of fluorosis are relying on an indication of an advanced form of the disease that has little to do with whether a person is suffering from joint pathologies caused by the earlier stages of fluorosis.
Conclusion
According to comprehensive data that has never before been considered by public health officials in the English-speaking world, it can reasonably be concluded that:
- Fluoride can cause osteoarthritis;
- The current limits on fluoride exposure in the United States permit levels of exposure that exceed the levels that cause significant bone and joint disease in China;
- The criterion that U.S. public health officials still use for detecting and preventing fluoride bone disease (i.e., x-ray evidence of fluorosis in the spine) represents a late stage in the course of fluoride poisoning and will not protect those patients who suffer fluoride-induced joint pathologies in the absence of telltale evidence of fluorosis.
If the word “safe” is to mean anything, current U.S. safety standards for fluoride need to be seriously reassessed in light of these findings. Until such a reassessment occurs, there can be no confidence that the grossly outdated standards now in place are able to prevent significant, and possibly widespread, harm to people’s bones and joints in the United States.
Further Information:
- Fluoride & Osteoarthritis
- Skeletal Fluorosis: The Misdiagnosis Problem
- Estimated Dose that Causes Skeletal Fluorosis
- Water Fluoride Levels that Cause Skeletal Fluorosis in India/China
References:
Bao W, et al. (2003). Report of investigations on adult hand osteoarthritis in Fengjiabao Village, Asuo Village, and Qiancheng Village. Chinese Journal of Endemiology 22(6):517-18. [FAN’s translation]
Cao J, et al. (2003). Brick tea fluoride as a main source of adult fluorosis. Food and Chemical Toxicology 41(4):535-42.
Chen X. (1988): Radiological Analysis of Fluorotic Elbow Arthritis. Journal of Guiyang Medical College 13(2):303-305. [FAN’s translation]
Expert Group of the Ministry of Health for the Study of Tea-Induced Fluoride Poisoning. (2000). The dose-response relationship of tea-induced osteofluorosis and brick tea fluoride intake. Chinese Journal of Endemiology 19(4):266-68. [FAN’s translation]
Ge X, et al. (2006). Investigations on the occurrence of osteoarthritis in middle-aged and elderly persons in fluorosis-afflicted regions of Gaomi City with high fluoride concentration in drinking water. Preventive Medicine Tribune 12(1):57-58. [FAN’s translation]
Institute of Medicine. (1997). Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride National Academy Press, Washington D.C. pp. 307-10.
Luo R, et al. (2012). Total knee arthroplasty for the treatment of knee osteoarthritis caused by endemic skeletal fluorosis. Chinese Journal of Tissue Engineering Research. Available online at: http://en.cnki.com.cn/Article_en/CJFDTOTAL-XDKF201209015.htm
National Research Council. (2006). Fluoride in drinking water: a scientific review of EPA’s standards. National Academies Press, Washington D.C. p. 178.
Su WM, et al. (2012). Total hip arthroplasty for the treatment of severe hip osteoarthritis due to fluorosis. Chinese Journal of Tissue Engineering Research 16(9):1543-1546. [See study]