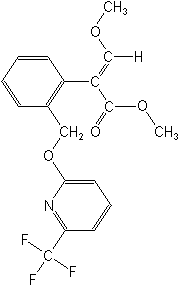
Note from FAN: Molecular formula for Picoxystrobin CAS No. 117428-22-5
ABSTRACT: The conclusions of EFSA following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State Czech Republic and co-rapporteur Member State Romania for the pesticide active substance picoxystrobin and the assessment of applications for maximum residue levels (MRLs) are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012. The conclusions were reached on the basis of the evaluation of the representative use of picoxystrobin as a fungicide on wheat (including spelt), triticale, barley, oats and rye. MRLs were assessed in oilseed rape and sunflower. The reliable end points, appropriate for use in regulatory risk assessment and the proposed MRLs, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
SUMMARY: … The conclusions laid down in this report were reached on the basis of the evaluation of the representative uses of picoxystrobin as a fungicide on wheat (including spelt), triticale, barley, oats and rye, as proposed by the applicant. MRLs were assessed in oilseed rape and sunflower. Full details of the representative uses and the proposed MRLs can be found in Appendix A of this report.
Data were submitted to conclude that the uses of picoxystrobin according to the representative uses proposed result in a sufficient fungicidal efficacy against the target organisms.
A data gap was identified for an appropriate search of the scientific peer-reviewed open literature covering all pertinent metabolites of picoxystrobin. Furthermore, the search terms used in the literature search did not cover all ecotoxicological data requirements.
In the area of identity, physical and chemical properties and analytical methods, no data gaps were identified.
In the area of mammalian toxicology and non-dietary exposure, several data gaps and issues that could not be finalised were identified. As no conclusion on the genotoxic potential of picoxystrobin could be drawn, the setting of reference values and the finalisation of human health risk assessment could not be conducted leading to a critical area of concern. The groundwater metabolites were concluded as being relevant to groundwater. The clastogenic and aneugenic potential of the metabolite IN-H8612 found as residue cannot be excluded leading to a critical area of concern. The compliance of the toxicity studies compared to the technical specification and the relevance of impurities should be reconsidered once the genotoxic potential of picoxystrobin is properly addressed. Data gaps concerning the toxicological profile of metabolites, in vitro comparative metabolism studies and further data to address the endocrine disruption potential of picoxystrobin lead to issues that could not be finalised.
Plant and animal residue definitions for risk assessment could not be proposed pending submission of further data to address the toxicity of some metabolites. As toxicological reference values could not be proposed for the active substance, a consumer risk assessment could not be performed and therefore MRLs have not been proposed. Data gaps have been identified for the submission of a standard hydrolysis study. In the framework of the MRL application, additional data are required to derive an MRL on sunflower.
The data available on environmental fate and behaviour are sufficient to carry out the required environmental exposure assessments at the European Union level for the representative uses, with the notable exception that information was not available regarding the effect of water treatment processes on the nature of residues that may be present in surface water and groundwater at the point of abstraction for drinking water purposes. This results in it not being possible to finalise the consumer exposure and risk assessments. For the representative uses on spring cereals, all Forum for the Co-ordination of Pesticide Fate Models and their Use (FOCUS) groundwater scenarios were predicted to have 80th percentile annual average recharge concentrations moving below 1 m for metabolites (that with the available data have to be considered toxicologically relevant) above the 0.1 ?g/L parametric drinking water limit. For the representative uses on winter cereals, this was the case for geoclimatic situations represented by 7/9 FOCUS groundwater scenarios. Consequently, the parametric drinking water limit of 0.1 ?g/L was estimated to be exceeded in more than half pertinent FOCUS groundwater scenarios for all the representative uses evaluated for three metabolites that have been assessed as toxicologically relevant. This resulted in a critical area of concern being identified, regarding the potential for groundwater contamination by metabolites IN-QDY62, IN-QDY63 and IN-QDK50 under the vulnerable conditions represented by the FOCUS groundwater scenarios.
In the area of ecotoxicology, a number of data gaps were identified. Critical areas of concern were identified as a high risk could not be excluded for earthworm-eating mammals for the metabolite IN-QDY63, for aquatic organisms and for earthworms. The risk assessment could not finalised for fish-eating birds and mammals due to the lack of appropriate bioconcentration factor values for the pertinent metabolites.
See EU report: Peer review of the pesticide risk assessment of the active substance picoxystrobin
