The FDA will now require pharmaceuticals to update drug labels and medication guides for all antibacterial drugs containing Fluoroquinolone. The labels and guides must reflect the findings of ongoing studies that led to an FDA warning issued earlier this year. The FDA cautioned practitioners to prescribe fluoroquinolones only when no alternative treatments are an option.
Fluoroquinolones are a family of broad spectrum, systemic antibacterial agents and include FDA-approved fluoroquinolones such as levofloxacin (Levaquin), ciprofloxacin (Cipro), ciprofloxacin extended-release tablets, moxifloxacin (Avelox), ofloxacin and gemifloxacin (Factive). They are indicated for the treatment of several bacterial infections, including bacterial bronchitis, pneumonia, sinusitis, urinary tract infections, septicemia and intraabdominal infections, joint and bone infections, soft tissue and skin infections, typhoid fever, bacterial gastroenteritis, urethral and gynecological infections, and pelvic inflammatory disease and several other infectious conditions.
The new requirements come as a result of studies that determined “the regular use of fluoroquinolone antibacterial drugs can affect tendons, muscles, joints, nerves, and the central nervous system.”
The FDA report notes, “Some patients may experience a ‘pins and needles’ tingling or pricking sensation,” hallucinations, or even confusion.”
Fluoroquinolones have fluoride as a central part of the drug. Fluoride is a known neurotoxin, and drugs with an attached fluoride molecule are able to penetrate into very sensitive tissues, including your brain.
The ability to cross the blood-brain barrier is what makes fluoride such a potent neurotoxin. Fluoride also disrupts collagen synthesis, and can damage your immune system by depleting energy reserves and inhibiting antibody formation in your blood.
Investigations continue regarding fluoroquinolone antibacterial drugs. The FDA will continue to update the public with any additional information as it becomes available.
Patients are advised to contact their healthcare providers if they experience any serious side effects that may be related to the use of fluoroquinolone treatment. Healthcare providers are advised to “switch to a non-fluoroquinolone antibacterial drug to complete the patient’s treatment course.” The FDA encourages healthcare professionals to report all adverse events associated with fluoroquinolone treatments to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
-END-
Chemical Structures:
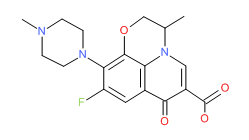
Ciprofloxacin HCl | CAS # 86393-32-0 | Molecular Formula: C17-H18-F-N3-O3.Cl-H.H2-O
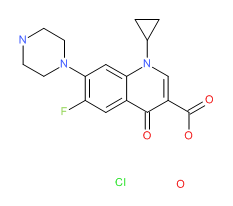
Gemifloxacin | CAS # 175463-14-6 | Molecular Formula: C18-H20-F-N5-O4
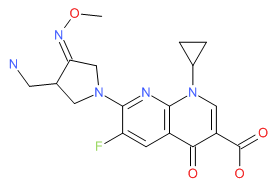
Levofloxacin | CAS # 100986-85-4 | Molecular Formula: C18-H20-F-N3-O4
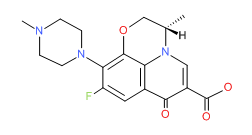
Moxifloxacin HCl | CAS # 186826-86-8 | Molecular Formula: C21-H24-F-N3-O4.Cl-H
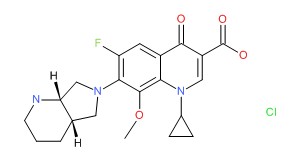
Norfloxacin | CAS # 70458-96-7 | Molecular Formula: C16-H18-F-N3-O3
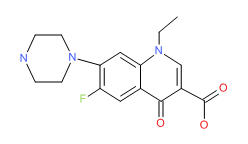
Ofloxacin | CAS # 82419-36-1 | Molecular Formula: C18-H20-F-N3-O4