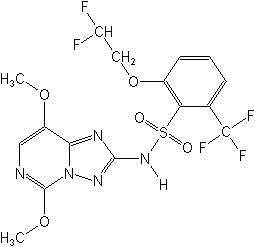
Molecular structure of Penoxsulam
*Online here
[Federal Register Volume 85, Number 46 (Monday, March 9, 2020)]
[Rules and Regulations]
[Pages 13548-13552]
From the Federal Register Online via the Government Publishing Office [www.gpo.gov]
[FR Doc No: 2020-04524]
———————————————————————–
ENVIRONMENTAL PROTECTION AGENCY
40 CFR Part 180
[EPA-HQ-OPP-2019-0061; FRL-10004-86]
Penoxsulam; Pesticide Tolerance
AGENCY: Environmental Protection Agency (EPA).
ACTION: Final rule.
———————————————————————–
SUMMARY: This regulation establishes a tolerance for residues of
penoxsulam in or on globe artichoke. Interregional Research Project
Number 4 (IR-4) requested this tolerance under the Federal Food, Drug,
and Cosmetic Act (FFDCA).
DATES: This regulation is effective March 9, 2020. Objections and
requests for hearings must be received on or before May 8, 2020, and
must be filed in accordance with the instructions provided in 40 CFR
part 178 (see also Unit I.C. of the SUPPLEMENTARY INFORMATION).
ADDRESSES: The docket for this action, identified by docket
identification (ID) number EPA-HQ-OPP-2019-0061, is available at http://www.regulations.gov or at the Office of Pesticide Programs Regulatory
Public Docket (OPP Docket) in the Environmental Protection Agency
Docket Center (EPA/DC), West William Jefferson Clinton Bldg., Rm. 3334,
1301 Constitution Ave. NW, Washington, DC 20460-0001. The Public
Reading Room is open from 8:30 a.m. to 4:30 p.m., Monday through
Friday, excluding legal holidays. The telephone number for the Public
Reading Room is (202) 566-1744, and the telephone number for the OPP
Docket is (703) 305-5805. Please review the visitor instructions and
additional information about the docket available at http://www.epa.gov/dockets.
FOR FURTHER INFORMATION CONTACT: Michael Goodis, Registration Division
(7505P), Office of Pesticide Programs, Environmental Protection Agency,
1200 Pennsylvania Ave. NW, Washington, DC 20460-0001; main telephone
number: (703) 305-7090; email address: RDFRNotices@epa.gov.
SUPPLEMENTARY INFORMATION
General Information
Does this action apply to me?
You may be potentially affected by this action if you are an
agricultural producer, food manufacturer, or pesticide manufacturer.
The following list of North American Industrial Classification System
(NAICS) codes is not intended to be exhaustive, but rather provides a
guide to help readers determine whether this document applies to them.
Potentially affected entities may include:
Crop production (NAICS code 111).
Animal production (NAICS code 112).
Food manufacturing (NAICS code 311).
Pesticide manufacturing (NAICS code 32532).
How can I get electronic access to other related information?
You may access a frequently updated electronic version of EPA’s
tolerance regulations at 40 CFR part 180 through the Government
Publishing Office’s e-CFR site at http://www.ecfr.gov/cgi-bin/text-idx?&c=ecfr&tpl=/ecfrbrowse/Title40/40tab_02.tpl.
How can I file an objection or hearing request?
Under FFDCA section 408(g), 21 U.S.C. 346a, any person may file an
objection to any aspect of this regulation and may also request a
hearing on those objections. You must file your objection or request a
hearing on this regulation in accordance with the instructions provided
in 40 CFR part 178. To ensure proper receipt by EPA, you must identify
docket ID number EPA-HQ-OPP-2019-0061 in the subject line on the first
page of your submission. All objections and requests for a hearing must
be in writing and must be received by the Hearing Clerk on or before
May 8, 2020. Addresses for mail and hand delivery of objections and
hearing requests are provided in 40 CFR 178.25(b).
In addition to filing an objection or hearing request with the
Hearing Clerk as described in 40 CFR part 178, please submit a copy of
the filing (excluding any Confidential Business Information (CBI)) for
inclusion in the public docket. Information not marked confidential
pursuant to 40 CFR part 2 may be disclosed publicly by EPA without
prior notice. Submit the non-CBI copy of your objection or hearing
request, identified by docket ID number EPA-HQ-OPP-2019-0061, by one of the following methods:
Federal eRulemaking Portal: http://www.regulations.gov.
Follow the online instructions for submitting comments. Do not submit
electronically any information you consider to be CBI or other
information whose disclosure is restricted by statute.
Mail: OPP Docket, Environmental Protection Agency Docket
Center (EPA/DC), (28221T), 1200 Pennsylvania Ave. NW, Washington, DC
20460-0001.
Hand Delivery: To make special arrangements for hand
delivery or delivery of boxed information, please follow the
instructions at http://www.epa.gov/dockets/contacts.html. Additional
instructions on commenting or visiting the docket, along with more
information about dockets generally, is available at http://www.epa.gov/dockets.
Summary of Petitioned-For Tolerance
In the Federal Register of May 9, 2019 (84 FR 20320) (FRL-9992-36),
EPA issued a document pursuant to FFDCA section 408(d)(3), 21 U.S.C.
346a(d)(3), announcing the filing of a pesticide petition (PP 8E8727)
by IR-4, Rutgers, The State University of New Jersey, 500 College Road
East, Suite 201W, Princeton, NJ 08540. The petition requested that 40
CFR 180.605 be amended by establishing a tolerance for residues of the
herbicide penoxsulam, including its metabolites and degradates, in or
on artichoke, globe at 0.01 parts per million (ppm). That document
referenced a summary of the petition prepared by Dow AgroSciences, the
registrant, which is available in the docket, http://www.regulations.gov. Comments were received on the notice of filing.
EPA’s response to these comments is discussed in Unit IV.C.
III. Aggregate Risk Assessment and Determination of Safety
Section 408(b)(2)(A)(i) of FFDCA allows EPA to establish a
tolerance (the legal limit for a pesticide chemical residue in or on a
food) only if EPA determines that the tolerance is “safe.” Section
408(b)(2)(A)(ii) of FFDCA defines “safe” to mean that “there is a
reasonable certainty that no harm will result from aggregate exposure
to the pesticide chemical residue, including all anticipated dietary
exposures and all other exposures for which there is reliable
information.” This includes exposure through drinking water and in
residential settings but does not include occupational exposure.
Section 408(b)(2)(C) of FFDCA requires EPA to give special
consideration to exposure of infants and children to the pesticide
chemical residue in establishing a tolerance and to “ensure that there
is a reasonable certainty that no harm will result to infants and
children from aggregate exposure to the pesticide chemical residue. . . .”
Consistent with FFDCA section 408(b)(2)(D), and the factors
specified in FFDCA section 408(b)(2)(D), EPA has reviewed the available
scientific data and other relevant information in support of this
action. EPA has sufficient data to assess the hazards of and to make a
determination on aggregate exposure for penoxsulam including exposure
resulting from the tolerances established by this action. EPA’s
assessment of exposures and risks associated with penoxsulam follows.
Toxicological Profile
EPA has evaluated the available toxicity data and considered its
validity, completeness, and reliability as well as the relationship of
the results of the studies to human risk. EPA has also considered
available information concerning the variability of the sensitivities
of major identifiable subgroups of consumers, including infants and
children.
The kidney was the major target organ for penoxsulam in the rat and
dog following subchronic and chronic dietary exposure. There are no
mechanistic studies characterizing the mode of action for renal
toxicity of penoxsulam or other triazolopyrimidine herbicides, but the
presence of crystals in the urinary tract and lack of tissue
bioaccumulation suggest that cellular inflammation and damage may occur
secondary to their presence. Hyperplasia (rat and dog) and inflammation
(rat) of the renal pelvic epithelium were observed by week 4 in dietary
dose range-finding studies. The dog was the more sensitive species in
studies of all durations. The rat, but not the dog, showed progression
of the severity of kidney toxicity with prolonged exposure. In dogs,
renal toxicity in the subchronic and chronic studies occurred at
comparable dose levels and measurable effects on renal function were
not observed. In the rat, effects on renal function (increased blood
urea nitrogen in both sexes, urinary bladder mucosal hyperplasia, and
increased severity of chronic glomerulonephropathy in males) were
observed only following chronic exposure, although the doses at which
kidney toxicity occurred were comparable to doses tested in the
subchronic study. A consistent pattern that identified a greater
sensitivity of either sex was not observed.
Other effects in the rat included decreased red blood cell
parameters and decreased body weight and/or weight gain. Liver effects
were observed at the higher dose levels in the dog 4-week feeding study
but not in other studies in the database. The findings of liver and/or
kidney effects are consistent with effects observed for other
triazolopyrimidine herbicides.
No effects of toxicological significance were observed in the
mouse. Penoxsulam showed no evidence of neurotoxicity or immunotoxicity
in the rodent, and no effects were seen in rats following dermal
exposure. The Agency waived the requirement for inhalation data based
on high inhalation margins of exposure using an oral endpoint, lack of
observed irritation effects, and low vapor pressure.
There was no evidence of increased pre- and/or post-natal
susceptibility. No developmental effects were observed in the rat or
rabbit. Maternal effects in the rat included decreased body weight gain
and food consumption and increased kidney weights. In the rabbit,
maternal effects included mortality, clinical signs of toxicity, and
decreased body weight gain and food consumption. In the rat 2-
generation reproductive toxicity study, delayed preputial separation
and lactation body weights were observed in F1 offspring at a dose that
caused kidney lesions in parental females.
Although there is evidence of an increased incidence of mononuclear
cell leukemia (MNCL) in Fisher 344 rats from exposure to penoxsulam,
EPA has concluded that a quantitative assessment of cancer is not
necessary and that the chronic reference dose (cRfD) is considered
protective of possible cancer effects.
Specific information on the studies received and the nature of the
adverse effects caused by penoxsulam as well as the no-observed-
adverse-effect-level (NOAEL) and the lowest-observed-adverse-effect-
level (LOAEL) from the toxicity studies can be found at http://www.regulations.gov in the document titled
“Penoxsulam: Human Health Risk Assessment for the Proposed Use on Globe Artichoke” (Penoxsulam HHRA) on pages 32-37 in docket ID number EPA-HQ-OPP-2019-0061. For
further discussion of the Agency’s rationale for its cancer conclusion,
see page 16 of the Penoxsulam HHRA.
Toxicological Points of Departure/Levels of Concern
Once a pesticide’s toxicological profile is determined, EPA
identifies toxicological points of departure (POD) and levels of
concern to use in evaluating the risk posed by human
exposure to the pesticide. For hazards that have a threshold below
which there is no appreciable risk, the toxicological POD is used as
the basis for derivation of reference values for risk assessment. PODs
are developed based on a careful analysis of the doses in each
toxicological study to determine the dose at which no adverse effects
are observed (the NOAEL) and the lowest dose at which adverse effects
of concern are identified (the LOAEL). Uncertainty/safety factors are
used in conjunction with the POD to calculate a safe exposure level–
generally referred to as a population-adjusted dose (PAD) or a
reference dose (RfD)–and a safe margin of exposure (MOE). For non-
threshold risks, the Agency assumes that any amount of exposure will
lead to some degree of risk. Thus, the Agency estimates risk in terms
of the probability of an occurrence of the adverse effect expected in a
lifetime. For more information on the general principles EPA uses in
risk characterization and a complete description of the risk assessment
process, see http://www2.epa.gov/pesticide-science-and-assessing-pesticide-risks/assessing-human-health-risk-pesticide.
A summary of the toxicological endpoints for penoxsulam used for
human risk assessment is discussed in Unit III.B of the final rule
published in the Federal Register of March 2, 2016 (81 FR 10771) (FRL-
9940-36).
Exposure Assessment
- Dietary exposure from food and feed uses. In evaluating dietary
exposure to penoxsulam, EPA considered exposure under the petitioned-
for tolerance as well as all existing penoxsulam tolerances in 40 CFR
180.605. EPA assessed dietary exposures from penoxsulam in food as
follows:
Acute exposure. Quantitative acute dietary exposure and risk
assessments are performed for a food-use pesticide, if a toxicological
study has indicated the possibility of an effect of concern occurring
as a result of a 1-day or single exposure.
No such effects were identified in the toxicological studies for
penoxsulam; therefore, a quantitative acute dietary exposure assessment
is unnecessary.
Chronic exposure. In conducting the chronic dietary exposure
assessment, EPA used the food consumption data from the United States
Department of Agriculture’s (USDA’s) 2003-2008 National Health and
Nutrition Examination Survey, What We Eat in America, (NHANES/WWEIA).
As to residue levels in food, the chronic dietary exposure assessment
was unrefined and used tolerance-level residues and 100 percent crop
treated (PCT).
iii. Cancer. Based on the data summarized in Unit III.A., EPA has
concluded that the cRfD is protective of potential cancer risk from
exposure to penoxsulam.
Anticipated residue and PCT information. EPA did not use
anticipated residue or PCT information in the dietary assessment for
penoxsulam. Tolerance-level residues and 100 PCT were assumed for all
food commodities as well as contribution to the 5-OH-penoxsulam
metabolite in fish.
- Dietary exposure from drinking water. The Agency used screening
level water exposure models in the dietary exposure analysis and risk
assessment for penoxsulam in drinking water. These simulation models
take into account data on the physical, chemical, and fate/transport
characteristics of penoxsulam. Further information regarding EPA
drinking water models used in pesticide exposure assessment can be
Penoxsulam is registered for control of aquatic weeds. For that use
pattern, the maximum application rate is 150 parts per billion (ppb) in
the water column. For the chronic dietary risk assessment, the water
concentration value of 150 ppb was used to assess the contribution to
drinking water. This value is likely to be an overestimate of actual
residues in drinking water.
- From non-dietary exposure. The term “residential exposure” is
used in this document to refer to non-occupational, non-dietary
exposure (e.g., for lawn and garden pest control, indoor pest control,
termiticides, and flea and tick control on pets).
Penoxsulam is currently registered for the following uses that
could result in residential exposures: Residential and commercial turf
(lawns and golf courses) and aquatic use sites. EPA assessed
residential exposure using the following assumptions: For handlers, it
is assumed that residential use will result in short-term (1 to 30
days) dermal and inhalation exposures. Residential post-application
exposure is also assumed to be short-term (1 to 30 days) in duration,
resulting from the following exposure scenarios:
Physical activities on turf: Adults (dermal) and children 1 to 2
years old (dermal and incidental oral);
Mowing turf: Adults (dermal) and children 11 to <16 years old
(dermal);
Exposure to golf courses during golfing: Adults (dermal), children
11 to <16 years old (dermal), and children 6 to <11 years old (dermal);
and
Exposure during aquatic activities (e.g. swimming): Adults (dermal,
inhalation, ingestion) and children 3 to <6 years old (dermal,
inhalation, ingestion).
Due to the lack of a dermal endpoint, EPA did not quantify exposure
and risk estimates from dermal exposure scenarios. EPA did not combine
exposure resulting from adult handler and post-application exposure
resulting from treated gardens, lawns, golfing, and/or aquatic areas in
residential settings because of the conservative assumptions and inputs
within each estimated exposure scenario. The Agency believes that
combining exposures resulting from handler and post-application
activities would result in an overestimate of adult exposure. EPA
selected the most conservative adult residential scenario (adult
handler inhalation exposure from backpack sprayer applications to
lawns/turf) as the contributing source of residential exposure to be
combined with the dietary exposure for the aggregate assessment. The
exposure for the aggregate assessment for children 3 to <6 years old is
based on post-application combined inhalation and ingestion exposures
during aquatic activities. The oral exposure for the aggregate
assessment for children 1 to <2 years old is based on post-application
hand-to-mouth exposures from applications to lawns/turf. To include
exposure from object-to-mouth and soil ingestion in addition to hand-
to-mouth would overestimate the potential for oral exposure. Further
information regarding EPA standard assumptions and generic inputs for
residential exposures may be found at http://www2.epa.gov/pesticide-science-and-assessing-pesticide-risks/standard-operating-procedures-residential-pesticide.
- Cumulative effects from substances with a common mechanism of
toxicity. Section 408(b)(2)(D)(v) of FFDCA requires that, when
considering whether to establish, modify, or revoke a tolerance, the
Agency consider “available information” concerning the cumulative
effects of a particular pesticide’s residues and “other substances
that have a common mechanism of toxicity.”
Unlike other pesticides for which EPA has followed a cumulative
risk approach based on a common mechanism of toxicity, EPA has not made
a common mechanism of toxicity finding as to penoxsulam and any other
substances and penoxsulam does not appear to produce a toxic metabolite produced
by other substances. For the purposes of this action, therefore, EPA
has not assumed that penoxsulam has a common mechanism of toxicity with
other substances. For information regarding EPA’s efforts to determine
which chemicals have a common mechanism of toxicity and to evaluate the
cumulative effects of such chemicals, see EPA’s website at
http://www2.epa.gov/pesticide-science-and-assessing-pesticide-risks/cumulative-assessment-risk-pesticides.
Safety Factor for Infants and Children
- In general. Section 408(b)(2)(C) of FFDCA provides that EPA
shall apply an additional tenfold (10X) margin of safety for infants
and children in the case of threshold effects to account for prenatal
and postnatal toxicity and the completeness of the database on toxicity
and exposure unless EPA determines based on reliable data that a
different margin of safety will be safe for infants and children. This
additional margin of safety is commonly referred to as the FQPA Safety
Factor (SF). In applying this provision, EPA either retains the default
value of 10X, or uses a different additional safety factor when
reliable data available to EPA support the choice of a different
factor.
- Prenatal and postnatal sensitivity. No evidence of quantitative
or qualitative increased susceptibility, as compared to adults, of rat
fetuses to in utero or postnatal exposure was observed in developmental
toxicity studies in rats or rabbits or a reproduction study in rats.
Developmental toxicity was not observed in the rat or rabbit up to
doses resulting in maternal toxicity. In the rat reproductive toxicity
study, slightly increased time to preputial separation in F1 males and
decreased pup weight gain were observed in the presence of parental
toxicity (kidney lesions in females).
- Conclusion. EPA has determined that reliable data show the
safety of infants and children would be adequately protected if the
FQPA SF were reduced to 1X. That decision is based on the following
findings:
- The toxicity database for penoxsulam is complete.
- There is no indication that penoxsulam is a neurotoxic chemical
and there is no need for a developmental neurotoxicity study or
additional uncertainty factors to account for neurotoxicity.
iii. There is no evidence that penoxsulam results in increased
susceptibility in in utero rats or rabbits in the prenatal
developmental studies or in young rats in the 2-generation reproduction
study.
- There are no residual uncertainties identified in the exposure
databases. The dietary food exposure assessments were performed based
on 100 PCT and tolerance-level residues. EPA made conservative
(protective) assumptions by using the high-end EDWC of 150 ppb from the
aquatic weed use pattern to assess exposure to penoxsulam in drinking
water. EPA used similarly conservative assumptions to assess post-
application exposure of children as well as incidental oral exposure of
toddlers. These assessments will not underestimate the exposure and
risks posed by penoxsulam.
Aggregate Risks and Determination of Safety
EPA determines whether acute and chronic dietary pesticide
exposures are safe by comparing aggregate exposure estimates to the
acute PAD (aPAD) and chronic PAD (cPAD). For linear cancer risks, EPA
calculates the lifetime probability of acquiring cancer given the
estimated aggregate exposure. Short-, intermediate-, and chronic-term
risks are evaluated by comparing the estimated aggregate food, water,
and residential exposure to the appropriate PODs to ensure that an
adequate MOE exists.
- Acute risk. An acute aggregate risk assessment takes into
account acute exposure estimates from dietary consumption of food and
drinking water. No adverse effect resulting from a single oral exposure
was identified and no acute dietary endpoint was selected. Therefore,
penoxsulam is not expected to pose an acute risk.
- Chronic risk. Using the exposure assumptions described in this
unit for chronic exposure, EPA has concluded that chronic exposure to
penoxsulam from food and water will utilize 5.6% of the cPAD for all
infants less than 1 year old, the population group receiving the
greatest exposure. Based on the explanation in Unit III.C.3., regarding
residential use patterns, chronic residential exposure to residues of
penoxsulam is not expected.
- Short-term risk. Short-term aggregate exposure takes into
account short-term residential exposure plus chronic exposure to food
and water (considered to be a background exposure level). Penoxsulam is
currently registered for uses that could result in short-term
residential exposure, and the Agency has determined that it is
appropriate to aggregate chronic exposure through food and water with
short-term residential exposures to penoxsulam.
Using the exposure assumptions described in this unit for short-
term exposures, EPA has concluded the combined short-term food, water,
and residential exposures result in aggregate MOEs of 5,500 for adults,
1,700 for children 1 to 2 years old, and 4,500 for children 3 to 5
years old. Because EPA’s level of concern for penoxsulam is a MOE of
100 or below, these MOEs are not of concern.
- Intermediate-term risk. Intermediate-term aggregate exposure
takes into account intermediate-term residential exposure plus chronic
exposure to food and water (considered to be a background exposure
level).
An intermediate-term adverse effect was identified; however,
penoxsulam is not registered for any use patterns that would result in
intermediate-term residential exposure. Intermediate-term risk is
assessed based on intermediate-term residential exposure plus chronic
dietary exposure. Because there is no intermediate-term residential
exposure and chronic dietary exposure has already been assessed under
the appropriately protective cPAD (which is at least as protective as
the POD used to assess intermediate-term risk), no further assessment
of intermediate-term risk is necessary, and EPA relies on the chronic
dietary risk assessment for evaluating intermediate-term risk for
penoxsulam.
- Aggregate cancer risk for U.S. population. As discussed in Unit
III.A., EPA has determined that an RfD approach based on the chronic
point of departure is appropriate for evaluating cancer risk. As there
are not chronic aggregate risks of concern, there are no cancer
aggregate risk concerns.
- Determination of safety. Based on these risk assessments, EPA
concludes that there is a reasonable certainty that no harm will result
to the general population, or to infants and children from aggregate
exposure to penoxsulam residues.
Other Considerations
Analytical Enforcement Methodology
Adequate enforcement methodologies using high performance liquid
chromatography with tandem mass spectroscopy (HPLC-MS/MS) are available
to enforce the tolerance expression. These methods may be requested
from: Chief, Analytical Chemistry Branch, Environmental Science Center,
701 Mapes Rd., Ft. Meade, MD 20755-5350; telephone number: (410) 305-
2905; email address: residuemethods@epa.gov.
International Residue Limits
In making its tolerance decisions, EPA seeks to harmonize U.S.
tolerances with international standards whenever possible, consistent
with U.S. food safety standards and agricultural practices. EPA
considers the international maximum residue limits (MRLs) established
by the Codex Alimentarius Commission (Codex), as required by FFDCA
section 408(b)(4). The Codex Alimentarius is a joint United Nations
Food and Agriculture Organization/World Health Organization food
standards program, and it is recognized as an international food safety
standards-setting organization in trade agreements to which the United
States is a party. EPA may establish a tolerance that is different from
a Codex MRL; however, FFDCA section 408(b)(4) requires that EPA explain
the reasons for departing from the Codex level.
The Codex has not established a MRL for penoxsulam on globe
artichoke.
Response to Comments
Two comments were received in response to the notice of filing. One
was against the Agency granting the use of penoxsulam and one was
against the use of pesticides in general. Although the Agency
recognizes that some individuals believe that pesticides should be
banned on agricultural crops, the existing legal framework provided by
section 408 of the Federal Food, Drug and Cosmetic Act (FFDCA)
authorizes EPA to establish tolerances when it determines that the
tolerance is safe. Upon consideration of the validity, completeness,
and reliability of the available data as well as other factors the
FFDCA requires EPA to consider, EPA has determined that these
penoxsulam tolerances are safe. The commenters have provided no
information to support an Agency conclusion that penoxsulam is not
safe.
Conclusion
Therefore, tolerances are established for residues of penoxsulam,
including its metabolites and degradates, in or on artichoke, globe at
0.01 ppm.
Statutory and Executive Order Reviews
This action establishes tolerances under FFDCA section 408(d) in
response to a petition submitted to the Agency. The Office of
Management and Budget (OMB) has exempted these types of actions from
review under Executive Order 12866, entitled “Regulatory Planning and
Review” (58 FR 51735, October 4, 1993). Because this action has been
exempted from review under Executive Order 12866, this action is not
subject to Executive Order 13211, entitled “Actions Concerning
Regulations That Significantly Affect Energy Supply, Distribution, or
Use” (66 FR 28355, May 22, 2001) or Executive Order 13045, entitled
“Protection of Children from Environmental Health Risks and Safety
Risks” (62 FR 19885, April 23, 1997), nor is it considered a
regulatory action under Executive Order 13771, entitled “Reducing
Regulations and Controlling Regulatory Costs” (82 FR 9339, February 3,
2017). This action does not contain any information collections subject
to OMB approval under the Paperwork Reduction Act (PRA) (44 U.S.C. 3501
et seq.), nor does it require any special considerations under
Executive Order 12898, entitled “Federal Actions to Address
Environmental Justice in Minority Populations and Low-Income
Populations” (59 FR 7629, February 16, 1994).
Since tolerances and exemptions that are established on the basis
of a petition under FFDCA section 408(d), such as the tolerance in this
final rule, do not require the issuance of a proposed rule, the
requirements of the Regulatory Flexibility Act (RFA) (5 U.S.C. 601 et
seq.), do not apply.
This action directly regulates growers, food processors, food
handlers, and food retailers, not States or Tribes, nor does this
action alter the relationships or distribution of power and
responsibilities established by Congress in the preemption provisions
of FFDCA section 408(n)(4). As such, the Agency has determined that
this action will not have a substantial direct effect on States or
Tribal Governments, on the relationship between the National Government
and the States or Tribal Governments, or on the distribution of power
and responsibilities among the various levels of government or between
the Federal Government and Indian Tribes. Thus, the Agency has
determined that Executive Order 13132, entitled “Federalism” (64 FR
43255, August 10, 1999) and Executive Order 13175, entitled
“Consultation and Coordination with Indian Tribal Governments” (65 FR
67249, November 9, 2000) do not apply to this action. In addition, this
action does not impose any enforceable duty or contain any unfunded
mandate as described under Title II of the Unfunded Mandates Reform Act
(UMRA) (2 U.S.C. 1501 et seq.).
This action does not involve any technical standards that would
require Agency consideration of voluntary consensus standards pursuant
to section 12(d) of the National Technology Transfer and Advancement
Act (NTTAA) (15 U.S.C. 272 note).
VII. Congressional Review Act
Pursuant to the Congressional Review Act (5 U.S.C. 801 et seq.),
EPA will submit a report containing this rule and other required
information to the U.S. Senate, the U.S. House of Representatives, and
the Comptroller General of the United States prior to publication of
the rule in the Federal Register. This action is not a “major rule”
as defined by 5 U.S.C. 804(2).
List of Subjects in 40 CFR Part 180
Environmental protection, Administrative practice and procedure,
Agricultural commodities, Pesticides and pests, Reporting and
recordkeeping requirements.
Dated: February 6, 2020. [published on March 7, 2020]
Michael Goodis,
Director, Registration Division, Office of Pesticide Programs.
Therefore, 40 CFR chapter I is amended as follows:
PART 180–[AMENDED]
- The authority citation for part 180 continues to read as follows:
Authority: 21 U.S.C. 321(q), 346a and 371.
- In Sec. 180.605, add alphabetically the entry “Artichoke, globe”
to the table in paragraph (a) to read as follows:
Sec. 180.605 Penoxsulam; tolerances for residues.
(a) * * *
————————————————————————
Parts per
Commodity million
————————————————————————
* * * * *
Artichoke, globe…………………………………….. 0.01
* * * * *
————————————————————————
[FR Doc. 2020-04524 Filed 3-6-20; 8:45 am]
BILLING CODE 6560-50-P
*Online here
