Note from the Fluoride Action Network:
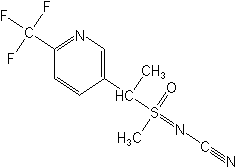
Molecular structure for Sulfoxaflor (see more information on its toxicity)
Excerpts
X11519540 (sulfonyl metabolite) approximately two times more toxic than parent sulfoxaflor
X11579457
X11596066 (ethyl metabolite)
X11718922 (identified in the Australian report)
X11719474
X11721061
PAGE 273-274: Toxicological data on metabolites
X11719474, the major soil and plant metabolite of sulfoxaflor, was of low acute oral toxicity in rats (LD50 > 5000 mg/kg bw) and showed no genotoxic potential in vitro in mammalian or microbial test systems. In a 90-day oral toxicity study in rats, the NOAEL was 1000 ppm (equal to 65.3 mg/kg bw per day), based on effects in the liver (vacuolization/fatty change) at 5000 ppm (equal to 327 mg/kgbw per day). In a reproduction toxicity screening study in rats, the NOAEL for reproductive and offspring performance was 5000 ppm (equal to 396 mg/kg bw per day), the highest dose tested. In a prenatal developmental toxicity study in rats, the NOAEL for developmental toxicity was 5000 ppm (equal to 368 mg/kg bw per day), the highest dose tested.
X11721061, a plant and animal (rat) metabolite of sulfoxaflor, was of low acute oral toxicity in rats (LD50 > 2000 mg/kg bw) and showed no genotoxic potential in vitro in mammalian or microbial test systems. In a 28-day oral toxicity study in rats, the NOAEL was 3000 ppm (equal to 236 mg/kg bw per day), based on reduced feed consumption at 8000 ppm (equal to 622 mg/kg bw per day).
X11596066, a metabolite of sulfoxaflor identified in hens and goats, was of low acute oral toxicity in rats (LD50 > 2000 mg/kg bw) and showed no genotoxic potential in vitro (Ames test).
X11579457, a soil metabolite of sulfoxaflor, was of low acute oral toxicity in rats (LD50 > 2000 mg/kg bw) and showed no genotoxic potential in vitro in mammalian or microbial test systems.
X11519540, a soil and animal (hen) metabolite of sulfoxaflor, was of moderate acute oral toxicity in rats (LD50 > 565 mg/kg bw) and showed no genotoxic potential in vitro in mammalian or microbial test systems. In a 28-day oral toxicity study in rats, the NOAEL was less than 100 ppm (equal to 7.7 mg/kg bw per day) in males, based on effects in liver (increased serum cholesterol), thyroid (follicular cell hypertrophy) and adrenals (increased vacuolization of the cortex).
All metabolites were less toxic than the parent compound, except for X11519540, which had higher acute and higher short-term toxicity than the parent.
There were no reports of adverse health effects in manufacturing plant personnel. Also, there were no reports of poisonings with sulfoxaflor.
The Meeting concluded that the existing database on sulfoxaflor was adequate to characterize the potential hazards to fetuses, infants and children.
PAGE 275:
Critical end-points for setting guidance values for exposure to sulfoxaflor
… Toxicologically significant compounds Sulfoxaflor, X11519540 (soil metabolite)
PAGE 278:
When rats were orally dosed with labelled sulfoxaflor, approximately 93% of the dose was eliminated in the urine and faeces as parent sulfoxaflor. The main metabolite in urine was a glucuronide conjugate of sulfoxaflor metabolite X11721061, accounting for approximately 2–4% of the dose. Several other unidentified minor components, each less than 1% of the administered dose, were present in the urine and faecal samples. Metabolism in laboratory animals was summarized and evaluated by the WHO panel of the JMPR in 2011.
When lactating goats were orally dosed with labelled sulfoxaflor at 12.2 ppm in the diet, approximately 4% of the dose appeared in the milk and 3% in the tissues.
Residue levels in milk reached a plateau during days 3–4 of the dosing phase, at about 0.2 mg/kg eq. 14C levels were highest in liver and kidney, and lowest in fat tissues. Parent sulfoxaflor was the predominant residue in all tissues, comprising over 89% TRR in milk, muscle, fat, and kidney; and approximately 60% TRR in liver. Metabolite X11519540 (sulfonyl metabolite) was found at low levels (? 0.009 mg/kg eq) in several matrices; while X11596066 (ethyl metabolite) was reported only in liver samples, at 18% TRR (0.095 mg/kg eq).Metabolite X11719474 was usually found at more than 10% TRR in plants, and consequently is considered a major plant metabolite. When lactating goats were orally dosed with labelled sulfoxaflor metabolite X11719474 at 11.4 ppm in the diet, approximately 1% of the dose appeared in the milk and 1% in the tissues.
Residue levels in milk reached a plateau during days 3–4 of the dosing phase, at about 0.2 mg/kg eq. Similar 14C levels were found in liver, kidney, milk, and muscle; while lower levels were reported in fat tissues. This study demonstrated that X11719474 is not metabolized in goats: no metabolites were identified, and the radioactivity found in all tissues was from X11719474.
When laying hens were dosed with labelled sulfoxaflor at 10.9 ppm in the feed, most of the dose was excreted in the droppings. Approximately 0.5% of the dose was recovered in the combined eggs, fat, and tissues. Residue levels in eggs reached a plateau during days 6–7 of the dosing phase, at about 0.06 mg/kg eq. 14C levels were highest in liver and lowest in fat tissues. Parent sulfoxaflor was the predominant residue in all tissues, comprising over 83% TRR in eggs, muscle, fat, and skin with fat; and approximately 68% TRR in liver. Metabolite X11519540 was found at low levels (? 0.005 mg/kg eq) in several matrices, while X11596066 was reported only in liver samples, at 14% TRR (0.020 mg/kg eq).
When laying hens were orally dosed with labelled sulfoxaflor metabolite X11719474 at 11.8 ppm in the feed, approximately 0.5% of the applied dose was recovered in the combined eggs, fat, and tissues. Similar 14C levels were noted in liver, muscle, and egg; lower levels were found in fat tissues. Approximately 92% of the dose was recovered from the excreta, and 0.3% in the cage rinse. Residue levels in eggs reached a plateau by day 4 of the dosing phase, with no compounds other than X11719474 being identified. This study demonstrated that X11719474 is not metabolized in hens: no metabolites were identified and the radioactivity found in all tissues was from X11719474.
PAGE 279: Animal metabolism summary
Metabolism studies in the laying hen and lactating goat demonstrated limited metabolism of sulfoxaflor and no metabolism of X11719474. In most hen and goat matrices, sulfoxaflor comprised approximately 80% or more of the total radioactivity when 14C-sulfoxaflor was fed. Metabolites X11721061 [? 0.017 mg/kg eq] and X11519540 [? 0.009 mg/kg eq] were found at < 10% TRR in several matrices. However, more extensive metabolism occurred in liver, with metabolite X11596066 comprising up to 18% TRR (0.095 mg/kg eq). Overall, the metabolism found in livestock was qualitatively similar to that observed in laboratory animals.
PAGE 283: Definition of the residue
… As sulfoxaflor was the major residue in all livestock commodities, the Meeting decided that, for animal commodities, parent sulfoxaflor is the appropriate residue of concern for MRL enforcement and dietary risk assessment. Although metabolite X11519540 is approximately two times more toxic than parent sulfoxaflor and is present in livestock commodities, however, it is present in such low levels (< 0.01 mg/kg) that it is not appropriate to include this metabolite in the residue definition.
*Read full report online at http://fluoridealert.org/wp-content/uploads/fao.sulfoxaflor-2011.pdf
See also:
• U.S. Federal Register, Sept 28, 2012, Sulfoxaflor; Pesticide Tolerances for Emergency Exemptions
• U.S. Federal Register, May 17, 2013, Sulfoxaflor; Pesticide Tolerances
• Australian 2013report: Evaluation of the New Active Constituent Sulfoxaflor in the Product Transform. Insecticide, Australian Pesticides and Veterinary Medicines Authority 2013
• FAO-WHO Maximum Residue Levels for food commodities
• 2015 paper by Dow scientists (Terry C, Rasoulpour RJ, Knowles S, Billington R. Utilizing relative potency factors (RPF) and threshold of toxicological concern (TTC) concepts to assess hazard and human risk assessment profiles of environmental metabolites: A case study, Regulatory Toxicology and Pharmacology, 71(2):301-317.
• June 26, 2013: U.S. §?180.668 Sulfoxaflor; tolerances for residues.
(a) General. Tolerances are established for residues of the insecticide sulfoxaflor, including its metabolites and degradates, in or on the commodities in the table. Compliance with the tolerance levels specified is to be determined by measuring only sulfoxaflor (N-[methyloxido[1-[6-(trifluoromethyl)-3-pyridinyl]ethyl]-?4-sulfanylidene]cyanamide).
Commodity |
Parts per million |
|---|---|
| Almond, hulls | 6.0 |
| Barley, grain | 0.40 |
| Barley, hay | 1.0 |
| Barley, straw | 2.0 |
| Bean, dry seed | 0.20 |
| Bean, succulent | 4.0 |
| Beet, sugar, dried pulp | 0.07 |
| Beet, sugar, molasses | 0.25 |
| Berry, low growing, subgroup 13-7G | 0.70 |
| Cattle, fat | 0.10 |
| Cattle, meat | 0.15 |
| Cattle, meat byproducts | 0.40 |
| Cauliflower | 0.08 |
| Citrus, dried pulp | 3.6 |
| Cotton, gin byproducts | 6.0 |
| Cotton, hulls | 0.35 |
| Cottonseed subgroup 20C | 0.20 |
| Fruit, citrus, group 10-10 | 0.70 |
| Fruit, pome, group 11-10 | 0.50 |
| Fruit, small, vine climbing, subgroup 13-07F, except fuzzy kiwi fruit | 2.0 |
| Fruit, stone, group 12 | 3.0 |
| Goat, fat | 0.10 |
| Goat, meat | 0.15 |
| Goat, meat byproducts | 0.40 |
| Grain, aspirated fractions | 20.0 |
| Grape, raisin | 6.0 |
| Hog, fat | 0.01 |
| Hog, meat | 0.01 |
| Hog, meat byproducts | 0.01 |
| Horse, fat | 0.10 |
| Horse, meat | 0.15 |
| Horse, meat byproducts | 0.40 |
| Leafy greens, subgroup 4A | 6.0 |
| Leafy petiole, subgroup 4B | 2.0 |
| Milk | 0.15 |
| Nuts, tree, group 14 | 0.015 |
| Onion, bulb, subgroup 3-07A | 0.01 |
| Onion, green, subgroup 3-07B | 0.70 |
| Pistachio | 0.015 |
| Poultry, eggs | 0.01 |
| Poultry, fat | 0.01 |
| Poultry, meat | 0.01 |
| Poultry, meat byproducts | 0.01 |
| Rapeseed, meal | 0.50 |
| Rapeseed subgroup 20A | 0.40 |
| Sheep, fat | 0.10 |
| Sheep, meat | 0.15 |
| Sheep, meat byproducts | 0.40 |
| Soybean, seed | 0.20 |
| Tomato, paste | 2.60 |
| Tomato, puree | 1.20 |
| Vegetable, brassica, leafy, group 5, except cauliflower | 2.0 |
| Vegetable, cucurbit, group 9 | 0.40 |
| Vegetable, fruiting, group 8-10 | 0.70 |
| Vegetable, leaves of root and tuber, group 2 | 3.0 |
| Vegetable, legume, foliage, group 7 | 3.0 |
| Vegetable, root and tuber, group 1 | 0.05 |
| Watercress | 6.0 |
| Wheat, forage | 1.0 |
| Wheat, grain | 0.08 |
| Wheat, hay | 1.5 |
| Wheat, straw | 2.0 |
(b) Section 18 emergency exemptions. [Reserved]
