Abstract
Fluorine is widely dispersed in nature and has multiple physiological functions. Although it is usually regarded as an essential trace element for humans, this view is not held universally. Moreover, chronic fluorosis, mainly characterized by skeletal fluorosis, can be induced by long-term excessive fluoride consumption. High concentrations of fluoride in the environment and drinking water are major causes, and patients with skeletal fluorosis mainly present with symptoms of osteosclerosis, osteochondrosis, osteoporosis, and degenerative changes in joint cartilage. Etiologies for skeletal fluorosis have been established, but the specific pathogenesis is inconclusive. Currently, active osteogenesis and accelerated bone turnover are considered critical processes in the progression of skeletal fluorosis. In recent years, researchers have conducted extensive studies in fields of signaling pathways (Wnt/B-catenin, Notch, PI3K/Akt/mTOR, Hedgehog, parathyroid hormone, and insulin signaling pathways), stress pathways (oxidative stress and endoplasmic reticulum stress pathways), epigenetics (DNA methylation and non-coding RNAs), and their inter-regulation involved in the pathogenesis of skeletal fluorosis. In this review, we summarised and analyzed relevant findings to provide a basis for comprehensive understandings of the pathogenesis of skeletal fluorosis and hopefully propose more effective prevention and therapeutic strategies.
Excerpt:
1. Introduction
Fluorine is one of the most common halogens, which usually exists in the environment as compounds. Fluorine or fluoride enters the human body primarily through drinking water, food, and air. Once absorbed into the bloodstream, it is easily transported throughout the body in the form of ions. More than 90% of absorbed fluoride is distributed in bone tissue, with the total amount of fluorine in a normal human body being approximately 2.6 g [1]. Fluoride has a dual effect on human well-being. Trace amounts of fluoride are required for the normal growth and development of the human body [2], while high intakes of fluoride can cause damage to tissues, organs, and systems. Dental fluorosis and skeletal fluorosis are characteristic manifestations of fluorosis. Long-term consumption of highly fluoridated water [2] and brick tea [3,4], or contaminated air and food entering the body owing to the use of high-fluoride coal as fuel [5,6] can lead to fluorosis. Unfortunately, there is no definite and effective treatment for patients with skeletal fluorosis, which severely threatens human health. Despite a declining incidence rate in recent years, fluorosis, particularly skeletal fluorosis, remains a severe public health problem in more than 40 countries worldwide. It is, therefore, crucial to recognize the mechanisms of skeletal fluorosis progression.
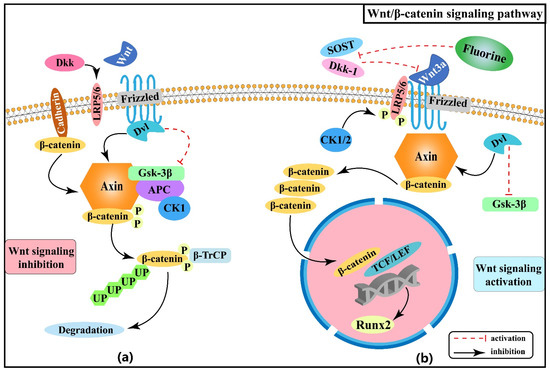
Fluorine has a strong bone affinity and tends to accumulate in bone tissue leading to skeletal fluorosis. Excessive fluoride will cause a series of damages to osteoblasts, osteoclasts, cartilage tissue, and bone mineralization in humans [7]. Fluorine has a bidirectional characteristic in bone production and resorption: it can not only cause osteosclerosis by enhancing osteogenic activity but also lead to osteoporosis by promoting bone resorption [8]. To date, the pathogenesis of skeletal fluorosis has not been fully explored. Over the years, researchers have focused on the various cellular regulatory mechanisms by which fluorine affects the bone turnover process [9]. Active osteogenesis and accelerated bone turnover have been proved to be crucial processes in the progression of skeletal fluorosis and the pathological basis for the diversity of osteogenic lesions [9,10]. Fluorine can induce differentiation and apoptosis of osteoblasts and osteoclasts, mainly by disrupting the dynamic balance of bone turnover, leading to skeletal damage and ultimately osteosclerosis, osteochondrosis, periosteal soft tissue ossification, osteoporosis, and degenerative changes in joints and cartilage in patients with skeletal fluorosis.
Researchers have made significant progress in understanding the regulation of signaling pathways, oxidative stress, endoplasmic reticulum stress, DNA methylation, and non-coding RNAs in recent years. By analyzing several studies on the relevant signaling pathways, stress pathways, epigenetics, and interaction networks among them involved in the pathogenesis of skeletal fluorosis, this review seeks to deepen understanding of the pathogenesis of fluorosis and hopefully propose more targeted prevention and therapeutic strategies.