Abstract
Epidemiological studies use biomarkers of fluoride exposure in pregnant women as surrogate measures of fetal fluoride exposure; however, there is little understanding of how pregnancy affects fluoride metabolism and its biomarkers. This narrative review summarizes the changes of pregnancy that have the potential to impact fluoride’s absorption, distribution and excretion, and highlights the limited body of evidence on the topic. The physiologic systems that experience pregnancy-associated changes relevant to fluoride’s metabolism are the cardiovascular, renal, metabolic and gastrointestinal, as well bone and calcium metabolism and the body’s acid-base balance. The available evidence indicates that fluoride is found in the maternal plasma and urine, placenta, amniotic fluid and fetus. Although plasma and urinary fluoride vary across gestation, there is insufficient quality evidence to determine the direction or extent of such variation. Furthermore, there is no doubt that fluoride from maternal blood crosses the placenta and is absorbed and excreted by the fetus; however, the biological mechanisms behind this placental passage are unknown. Research on maternal and prenatal biomarkers of fluoride exposure would benefit from studies on how pregnancy-associated changes affect the metabolism of fluoride across gestation, the mechanisms for the intestinal absorption of fluoride in pregnant women, and the placental passage of fluoride.
Keywords: fluoride; fluoride metabolism; fluoride pharmacokinetics; pregnancy; pregnant; maternal-fetal fluoride exchange
1. Introduction
The fluoride ion is ubiquitous in the environment [1], constitutes a trace element of the human diet [2] and is of particular interest in dentistry and public health. Fluoride is considered a case of nutritional hormesis, the concept that the ingestion of small quantities has the opposite effect to ingestion of large quantities [3]. Fluoridation of community water [4] or salt for human consumption [5] has been established as an effective strategy for the prevention of dental caries, with stronger evidence supporting its effectiveness in children, and fewer studies in adults [6]. In contrast, fluoride exposure has also been associated with detrimental effects in human bones, teeth [7] and more recently neurodevelopment [8]. Considering that the prenatal period has recently been identified as a potential risk window for fluoride exposure, the challenge for those working towards achieving a balance between fluoride’s benefits and risks depends on finding the dose of exposure at which benefits can be maintained while minimizing side effects in vulnerable populations.
The epidemiological studies reporting associations between prenatal fluoride exposure and health outcomes in children have used biomarkers of fluoride exposure in pregnant women [9]. However, the use of these biomarkers has a major limitation: our narrow understanding of fluoride metabolism during pregnancy and how it may affect biomarker levels. Ideally, the choice of biomarkers of prenatal exposure should be informed by knowledge on how fluoride is absorbed, distributed and excreted by the mother and the fetus; unfortunately, that knowledge is limited. Responding to this knowledge gap, this narrative review aims to summarize the limited available evidence on the metabolism of fluoride in pregnant women (for thorough reviews on the metabolism of fluoride in the general population please refer to Whitford [10] and Buzalaf and Whitford [11]). An overview of the physiological changes of pregnancy will be presented, and those that potentially modify the metabolism of fluoride will be identified and summarized. Then, a systematic search and critical analysis of the literature on the absorption, distribution, and excretion of fluoride in pregnant women is presented, together with perspectives for future research.
2. Overview of Pregnancy and Summary of Changes with the Potential to Impact Fluoride Metabolism
During the first trimester of pregnancy, most physiologic changes are secondary to hormonal responses triggered after fertilization. One of the first changes observed in the first trimester occurs in the cardiovascular system: a dramatic increase in plasma volume, ~50% above nonpregnant values [12]. The new demand for oxygen increases the production of red blood cells by about 30%; however, they become diluted due to plasma volume increasing at a higher rate (low hematocrit due to hemodilution) [13]. These cardiovascular changes then lead to renal adaptations: the increased blood volume delivered to the kidneys raises the glomerular filtration rate by 40–60%. As a result, there is both an increase in urine flow and volume, and the filtration and excretion of water and solutes [14]. These cardiovascular and renal changes have the potential to dilute plasma and urinary fluoride levels.
As pregnancy progresses, the physical and physiological demands increase the body’s metabolic rate, leading to higher energy needs and nutritional requirements [15]. Additionally, the increased secretion of the hormones progesterone and relaxin, loosen smooth muscle and decrease the motility of the gastrointestinal tract, causing constipation, heartburn and reflux [16]. Pregnant women usually alleviate these symptoms through dietary modifications and drugs. Dietary modifications may include decreased consumption of triggers (highly acidic foods such as coffee, tomatoes and carbonated beverages) and an increase in the intake of healthier, less acidic alternatives [17]. On the other hand, the pharmacological management of gastrointestinal symptoms includes the use of antiacids (e.g., calcium carbonate, aluminum or magnesium hydroxide) and acid reducers (e.g., famotinide, ranitidine and omeprazole) [18]. Therefore, during pregnancy there is potential for a higher intake of fluoride [19], but also for decreased absorption through the stomach and small intestine, either because of decreased stomach acidity or increased consumption of calcium-containing supplements.
Around the second trimester, when the fetus’ presence becomes more evident, most physiological changes are secondary to fetal size. The gradual size-increase of the uterus pushes up the diaphragm up to 4 cm above its usual position, diminishing total lung capacity [16]. To compensate for the lower lung capacity, progesterone acts as a respiratory stimulant, increasing the volume of air inhaled per minute and leading to a state of hyperventilation—breathing faster and deeper [20]. The increased ventilation responds to the fetal demand for oxygen, but the increased exhalation of carbon dioxide (CO2) leads to disturbances in the body’s acid-base balance and the blood’s bicarbonate (HCO?3) buffering system: as more CO2 is lost, hydrogen (H+) ions are removed and blood acidity decreases, leading to chronic respiratory alkalosis [21]. To compensate for the decrease in blood H+, the kidneys excrete HCO?3 through the urine and retain H+ to maintain the blood’s pH at physiological levels [16]. This means that there may be a slightly more alkaline urine during pregnancy [22], a factor that has the potential to increase the urinary excretion of fluoride [11].
By the end of the second trimester, the intestinal absorption of calcium has doubled. This increased absorption allows for the buildup of maternal skeletal calcium stores to meet fetal demands during the third trimester [16]. Therefore, as pregnancy progresses, bone metabolism transitions from a state of predominantly maternal bone formation, increased bone density and calcium storage, to increased bone turnover for the transfer of calcium to the fetus towards the end of gestation [23]. The increased bone turnover of pregnancy has the potential to also release skeletal stores of fluoride.
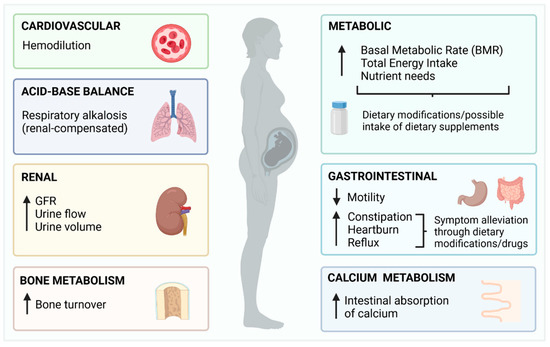
Overall, pregnancy induces changes in all major physiological systems [16], with some potentially affecting the absorption, distribution and excretion of fluoride. A summary of these physiological changes is depicted in Figure 1.
Figure 1. Summary of the physiological changes of pregnancy with potential to affect fluoride metabolism.
3. Available Evidence on the Absorption, Distribution, and Excretion of Fluoride in Pregnant Women
3.1. Literature Search
The literature search was conducted in two databases through the Ovid search interface: MEDLINE (1946 to 30 December 2021) and EMBASE (1974 to 30 December 2021), using a subject-heading approach. MEDLINE’s subject headings utilized were “Fluorides [Metabolism, Pharmacokinetics, Pharmacology, Physiology, Urine] AND Pregnancy or maternal-fetal exchange”. EMBASE’s identified subject headings were “Fluoride or fluoride ion AND fetomaternal transfusion”. The inclusion criteria were original investigations conducted in humans and reported in English language. The exclusion criteria were articles for which the full text was not available, articles which focused on a different topic (e.g., dental caries) or were grey literature. To remove duplicates, the citations from the papers that met inclusion and exclusion criteria from both databases, were exported to EndNote 20®. From the final articles retrieved from both databases (n = 29), additional references were identified by a manual search among the cited references (n = 12), adding to a total of 41 original articles critically reviewed for the following sections.
3.2. Fluoride Absorption in Pregnant Women
There is no data on the absorption of fluoride specifically gathered from pregnant women. In nonpregnant adults, and when ingested in the absence of inhibitors (such as food and calcium-containing products) [24], approximately 25% of the absorption of fluoride occurs in the stomach through a pH-dependent mechanism [25]. The remaining 75% of absorption occurs in the proximal small intestine through a pH-independent mechanism—via paracellular channels [26]. Whether these proportions and mechanisms are affected by the physiological adaptations and dietary modifications associated with pregnancy is unknown, and studies addressing these questions need to be conducted.
3.3. Distribution of Fluoride in Pregnant Women
3.3.1. Maternal Blood
Fluoride in whole blood is the sum of ionic and nonionic fluoride. Ionic fluoride is the measure of significance for the health sciences and public health, and the one available to participate in biological reactions [11]. Under steady-state conditions (pH = 7.4 and normal hematocrit), ionic fluoride is asymmetrically distributed between plasma and blood cells in a 2:1 proportion [27]. Plasma is the central compartment for the distribution of fluoride. The levels of fluoride in plasma are not homeostatically regulated, and fluctuate depending on dietary and environmental exposure, exchange with soft and hard tissues, and urinary excretion. For this reason, plasma-fluoride levels have been used as a biomarker of present fluoride exposure [28]. In nonpregnant adults, fasting plasma-fluoride levels have been reported over a wide range, between 0.009 and 0.66 mg/L [28].
The first studies on fluoride levels in maternal plasma were motivated by the interest in the placental passage of fluoride, and the hypothesis that ingested fluoride could prenatally incorporate into developing enamel to make it resistant to dental caries [29]. As research evolved to conclude that the mechanism for the protective effect of fluoride on dental caries is not through prenatal incorporation into dental enamel but by topical exposure after tooth eruption [30], a gap arose in studies on maternal plasma fluoride levels (from the 1980s to the early 2000?s). Recently, there has been a renewed interest in this topic with studies using maternal plasma fluoride as a biomarker of prenatal exposure. A summary of available reports of plasma or serum fluoride levels in pregnant women [27,28,29,30,31] is displayed in Table 1. From this table, it can be inferred that reported plasma fluoride levels in pregnant women are within the range reported for nonpregnant adults [28]; however, two observations can be made: (1) as in nonpregnant people, maternal plasma/serum fluoride levels seem to be higher in areas with higher levels of fluoride in community fluoridation programs; and (2) fluoride levels seem to be lower compared to nonpregnant women and to decrease towards the end of gestation. The latter observation has been often interpreted as evidence of an association with increased fetal fluoride uptake at the time of fetal bone mineralization [31,32]. This assumption, however, ignores the physiologic hemodilution that occurs towards term. Interestingly, hemodilution peaks at 32 weeks [16], precisely when fluoride levels in pregnant women have been reported at their lowest. Furthermore, the changes in the acid-base balance that occur during pregnancy (such as physiologic hyperventilation or the metabolic acidosis associated with cases of gestational diabetes), lead to variations in blood pH that could potentially affect plasma or serum fluoride levels. To rule out the confounding effect of hemodilution in studies assessing plasma fluoride levels in pregnant women, future studies should control for this factor utilizing individual measures of hematocrit and consider gestational age and any condition involving disturbances in the body’s acid-base balance [27]. Whether the decrease in plasma and serum fluoride levels observed towards term is an artifact of changes in hemodynamics or the pregnant body’s acid-base balance, remains to be determined.
Table 1. Summary of reports of plasma or serum fluoride levels in pregnant women.

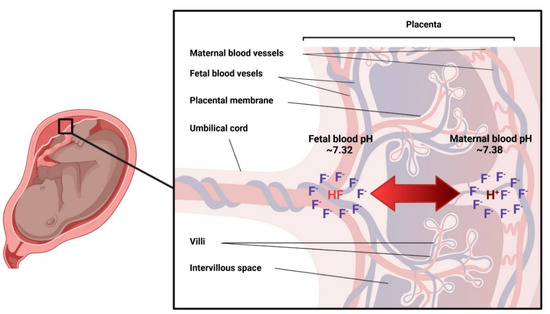
3.3.2. Placenta
The placenta is a complex organ that constitutes an interface between the mother and fetus [16], and has metabolic, endocrine, immunologic and transport functions [32]. Fluoride has been measured in the placenta with high variability within and between samples [33]. Placental fluoride levels also vary depending on the sampling area, with higher concentrations in the placental periphery (probably as part of calcium precipitates that form in the periphery towards term) [34] and in placentas from women who had been supplemented with fluoride tablets or were living in fluoridated areas [34,35,36]. One study conducted in humans using radioactive fluoride, found that fluoride levels in pre-term placentas were lower than those of maternal plasma [37]. In contrast, studies in placentas collected at term, report higher levels than in the plasma [34,35,36]. The available evidence, therefore, suggests that fluoride is found in the placenta and its levels depend on exposure, placental sampling area, and gestational age.