Abstract
Highlights
- • Fluoride may disrupt thyroid function.
- • Thyroid dysfunction in pregnancy can adversely impact offspring development.
- • Fluoride in water increased risk of hypothyroidism in pregnant women.
- • Boys had lower IQ scores if their mothers were hypothyroid in pregnancy.
- • Thyroid disruption may contribute to developmental neurotoxicity of fluoride.
FAN Note: This study is the strongest to date showing that fluoride causes hypothyroidism and also that hypothyroidism in the mother is associated with lower IQ in the child. See the York University press release on the study here.
Background
While fluoride can have thyroid-disrupting effects, associations between low-level fluoride exposure and thyroid conditions remain unclear, especially during pregnancy when insufficient thyroid hormones can adversely impact offspring development.
Objectives
We evaluated associations between fluoride exposure and hypothyroidism in a Canadian pregnancy cohort.
Methods
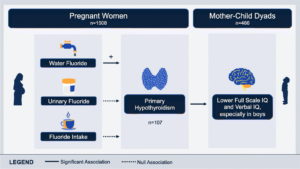
We measured fluoride concentrations in drinking water and three dilution-corrected urine samples and estimated fluoride intake based on self-reported beverage consumption. We classified women enrolled in the Maternal-Infant Research on Environmental Chemicals Study as euthyroid (n = 1301), subclinical hypothyroid (n = 100) or primary hypothyroid (n = 107) based on their thyroid hormone levels in trimester one. We used multinomial logistic regression to estimate the association between fluoride exposure and classification of either subclinical or primary hypothyroidism and considered maternal thyroid peroxidase antibody (TPOAb) status, a marker of autoimmune hypothyroidism, as an effect modifier. In a subsample of 466 mother-child pairs, we used linear regression to explore the association between maternal hypothyroidism and child Full-Scale IQ (FSIQ) at ages 3-to-4 years and tested for effect modification by child sex.
Results
A 0.5 mg/L increase in drinking water fluoride concentration was associated with a 1.65 (95 % confidence interval [CI]: 1.04, 2.60) increased odds of primary hypothyroidism. In contrast, we did not find a significant association between urinary fluoride (adjusted odds ratio [aOR]: 1.00; 95%CI: 0.73, 1.39) or fluoride intake (aOR: 1.25; 95%CI: 0.99, 1.57) and hypothyroidism. Among women with normal TPOAb levels, the risk of primary hypothyroidism increased with both increasing water fluoride and fluoride intake (aOR water fluoride concentration: 2.85; 95%CI: 1.25, 6.50; aOR fluoride intake: 1.75; 95%CI: 1.27, 2.41). Children born to women with primary hypothyroidism had lower FSIQ scores compared to children of euthyroid women, especially among boys (B coefficient: ?8.42; 95 % CI: ?15.33, ?1.50).
Discussion
Fluoride in drinking water was associated with increased risk of hypothyroidism in pregnant women. Thyroid disruption may contribute to developmental neurotoxicity of fluoride.
Full Text:
1. Introduction
Fluoride is added to some public water supplies and dental products to prevent dental caries (Community Water Fluoridation, 2019). Drinking water is the largest source of fluoride for children and adults living in fluoridated communities, accounting for 40 %–70 % of daily intake (United States Environmental Protection Agency, n.d.). Black tea is another dietary source of fluoride (Krishnankutty et al., 2021). Questions about systemic fluoride exposure during pregnancy have increased following findings from studies linking gestational exposure to fluoride with neurodevelopmental deficits, including lower intelligence quotient (IQ) (Bashash et al., 2017; Cantoral et al., 2021; Green et al., 2019).
Disruption of thyroid hormone is a potential mechanism underlying developmental fluoride neurotoxicity (National Toxicology Program, n.d.; National Research Council, 2006), especially during the first 10–12 weeks of gestation when the fetus is exclusively reliant on maternal thyroid hormones (Andersen et al., 2013; de Escobar et al., 2004; Chevrier et al., 2011). Maternal thyroid hormone insufficiency (i.e., hypothyroidism) early in gestation is associated with adverse effects on offspring development, including preterm birth (Andersen et al., 2013) and diminished IQ (Andersen et al., 2018; Haddow et al., 1999). A meta-analysis of three prospective birth cohorts found that maternal hypothyroxinemia (i.e., normal thyroid stimulating hormone [TSH] and low [<2.5th percentile] free thyroxine [FT4]) was associated with a 3 to 4-point reduction in offspring IQ (Levie et al., 2018).
While thyroid-disrupting effects of fluoride have been known since the 1930s (Day and Powell-Jackson, 1972), evidence of an association between low-level exposure to fluoride and thyroid disruption is mixed with few studies conducted in pregnancy. In experimental studies, Wistar rats whose mothers were exposed in pregnancy to higher levels of fluoride (i.e., 20 mg/kg of body weight (Banji et al., 2013) and >100 ppm (Basha et al., 2011)) showed decreases in serum FT4 and free triiodothyronine (FT3). Similar findings were reported in female and male Wistar rats at lower, prolonged fluoride exposure levels (Jiang et al., 2016), but not in Long-Evans hooded male rats (McPherson et al., 2018), perhaps reflecting strain differences in response to fluoride
exposure (Kang et al., 1986).
In children and adults, higher fluoride levels in drinking water and in urine were associated with elevated serum TSH and lower serum free and total T4 and T3 levels, considered characteristic of hypothyroidism (Khandare et al., 2018; Kheradpisheh et al., 2018; Wang et al., 2020). Likewise, in a population-based study conducted in England, hypothyroidism was 1.6 times more likely among adults living in areas with water fluoride levels >0.7 mg/L compared to areas with water-fluoride concentrations <0.3 mg/L (Peckham et al., 2015). In contrast, an observational study (Barberio et al., 2017) conducted in Canada reported no association between fluoride exposure and thyroid hormone levels or self-reported diagnosis of a thyroid condition. However, when iodine status was considered, an association between water fluoride concentration and TSH was found among iodine deficient individuals (Malin et al., 2018). A 2018 systematic review also reported a positive relationship between fluoride exposure and hypothyroidism in children and adults (Chaitanya et al., 2018), though this association was limited to a small number of ecological and cross-sectional studies.
To date, no studies have assessed the association between fluoride exposure and thyroid function in pregnant women living in areas with optimally fluoridated water. If fluoride is associated with a reduction in thyroid hormone, thereby reducing the availability of the hormone to the fetus, then fluoride could affect offspring cognitive function. We evaluated the potential thyroid-disrupting effects of fluoride exposure in pregnancy and explored whether hypothyroidism in pregnancy was associated with children’s IQ among Canadian mother-child dyads.
2. Methods
2.1. Participants
Pregnant women were enrolled in the Maternal-Infant Research on Environmental Chemicals (MIREC) Study (Arbuckle et al., 2013) between 2008 and 2011 from ten cities across Canada, seven of which add fluoride to drinking water (Toronto, Hamilton, Ottawa, Sudbury, Halifax, Edmonton, Winnipeg) and three of which do not (Vancouver, Montreal, Kingston). Women were eligible to participate if they were ?18 years of age, able to communicate in English or French, and <14 weeks’ gestation. Participants were considered ineligible if they had known fetal abnormalities, medical complications, or reported drug use. Of 2001 women recruited, 1983 consented to participate; of these, 1885 (95.1 %) provided plasma samples in trimester one.
Mothers of singleton children born >28 weeks’ gestation who were between the ages of 3–4 years of age at time of study, with no congenital abnormalities, major neurological disorders, or history of convulsions were contacted to participate with their children in a neurodevelopmental follow-up study (i.e., MIREC-Child Development Plus). Owing to limited resources, in-person IQ testing was offered in six study sites (Toronto, Hamilton, Halifax, Vancouver, Kingston, and Montreal). Of 1207 eligible women, 808 consented to participate in this follow-up study; of these, 610 (76 %) agreed to child neurodevelopmental testing and 601 completed IQ testing in entirety.
The current study received approval from the research ethics boards at Health Canada and York University. All participants provided written informed consent at time of enrollment in MIREC and MIREC-Child Development Plus.
2.2. Maternal fluoride exposure
2.2.1. Water fluoride (mg/L)
Municipal drinking water reports from 2008 to 2012 were solicited from municipal water treatment plants (WTPs) in all ten cities included in MIREC. These reports listed water fluoride concentrations that were measured daily if fluoride was added to public drinking water, and weekly or monthly if fluoride was not added to public water (Till et al., 2018). Using the first three letters of their postal code, participants’ residences were matched with boundary regions serviced by each WTP. Average water fluoride concentration (i.e., geometric mean; mg/L) was estimated for each woman who reported drinking tap water in pregnancy by averaging water fluoride concentrations across each woman’s pregnancy; thus, each woman has a water fluoride concentration that is matched in time to the levels of fluoride found in tap water for the duration of her pregnancy. Further details can be found in Till et al. (Till et al., 2018).
2.2.2. Maternal fluoride intake (mg/day)
We collected information on women’s consumption of tap water, tea, and coffee from a self-reported questionnaire completed in the first and third trimesters. We estimated daily maternal fluoride intake (mg/day) in trimester one and three separately by multiplying water fluoride concentration (mg/L) by total volume (L) of water, tea, and coffee consumed. Volume of water and water-based beverages was only derived for women who reported drinking tap water in pregnancy. We then added the additional fluoride content that would be expected from drinking each 200-mL cup of black tea (0.326 mg) or green tea (0.260 mg) if made with deionized water (Krishnankutty et al., 2021). Estimated maternal dietary fluoride intake (mg/day) was derived by taking the average of fluoride intake for trimesters one and three. See Supplemental Material, Appendix A for the formula used to derive maternal fluoride intake.
2.2.3. Maternal urinary fluoride (MUF; mg/L)
MUF concentration was analyzed in spot urine samples collected in each trimester of pregnancy, using a modification of the hexamethyldisiloxane (HMDS; Sigma Chemical Co., USA) microdiffusion method with ion-selective electrode by the Indiana University School of Dentistry (Martínez-Mier et al., 2011). The limit of detection (LoD) was 0.02 mg/L; trimester-specific concentrations below the LoD were replaced with the value of LoD/?2 (n = 23), which is a validated method for estimation of the average concentration from data containing nondetectable values (Hornung and Reed, 1990). Each MUF concentration was standardized for urine specific gravity (SG) to account for variability due to urinary dilution using the following equation: MUFSG = MUF × [(SGM ? 1) ÷ (SGi ? 1)], where MUFSG is the SG-adjusted fluoride concentration (mg/L), SGi is the observed SG concentration for the individual urine sample, and SGM is the median SG for the cohort (Duty et al., 2005). We derived the average dilution-adjusted MUFSG concentration by taking the average across all three trimesters for each woman. We removed one averaged MUFSG value (>5 mg/L) because of uncertainty that it reflected an individual’s true exposure; this high MUFSG concentration was driven by one trimester-specific value of 16 mg/L, which was not consistent with the other trimester values that were close to zero. Thus, this extreme value was more likely to represent fluoride ingestion (e.g., swallowing toothpaste prior to urine sample) rather than a reliable exposure measure.
For comparison, we also used urinary creatinine to correct for dilution when measuring MUF. Urinary creatinine was measured using a colorimetric end-point assay (Jaffe) on an Indiko instrument (Indiko Plus, ThermoFisher Scientific). An alkaline sodium picrate solution was used to react with creatinine in urine to form a red Janovski complex using Mircogenics DRI® Creatinine-Detect® Test. The absorbance was read at 510 nm on an Indiko chemistry autoanalyzer with a detection limit of 0.069 mmol/L, reporting limit of 0.23 mmol/L, and reproducibility of 2.2 %. While both methods are commonly used to correct for urine dilution with little difference observed between the two methods (Till et al., 2018), we chose to use MUFSG for our primary model because of the larger sample size relative to creatinine-adjusted MUF.
2.3. Classification of thyroid status
TSH and FT4 were analyzed in maternal plasma samples from the first trimester (mean [standard deviation (SD)] = 11.6 (1.6) weeks). Plasma FT4 was measured using gold standard equilibrium dialysis isotope dilution mass spectrometry (ED-ID-MS) by the accredited Toxicology Laboratory at the Institut National de Santé Publique du Québec (INSPQ). Plasma FT4 was considered normal if it fell between the 10th and 90th percentiles of FT4 levels for all women in MIREC (i.e., 11–17 pg/mL). Plasma TSH was quantified using a commercial immunoassay by an accredited biochemistry laboratory at the Institut Universitaire de Cardiologie et de Pneumologie de Québec (IUCPQ) and compared against the trimester one reference range as recommended by the American Thyroid Association (Stagnaro-Green et al., 2011) (i.e., 0.1–2.5 ?IU/mL). The LoD was 0.0025 ?IU/mL; concentrations below the LoD (n = 7) were given a value of LoD/?2 (Hornung and Reed, 1990). We also measured thyroid peroxidase antibody (TPOAb) levels, a marker of autoimmune hypothyroidism. Elevated TPOAb levels (considered in this study as ?5.61 IU/mL based on lab protocols from the IUCPQ), which have been associated with a reduced capacity to regulate thyroid hormones due to autoimmune destruction of the thyroid gland, have been shown to modify the association between per– and polyfluoroalkyl substances and thyroid hormones in pregnant women (Webster et al., 2014).
Women were classified as euthyroid if their TSH and FT4 levels fell within the population reference range (i.e., 0.1–2.5 ?IU/mL and 11–17 pg/mL, respectively), and subclinical hypothyroid if their TSH levels were above the top limit of the reference range but FT4 levels were within range (i.e., 2.5–10 ?IU/mL (Stagnaro-Green and Pearce, 2012) and 11–17 pg/mL, respectively). Notably, women were classified as primary hypothyroid if their TSH levels were high and FT4 levels were low (i.e., >2.5 ?IU/mL and < 11 pg/mL, respectively), or if they had reported a prior clinical diagnosis of hypothyroidism. Women meeting criteria for hyperthyroidism (subclinical or primary) were excluded from all analyses.
2.4. Measures of iodine status
Thyroglobulin (Tg) was measured by the accredited Toxicology Laboratory at the INSPQ using an Abbott Architect i2000SR immunoassay analyzer. Tg was used as a biomarker of long-term iodine nutrition (Ma and Skeaff, 2014), which was used as a covariate in sensitivity analyses. The LoD was 0.09 ?g/L. In addition, we describe urinary iodine adjusted for creatinine (UIC/Cr) as well as daily iodine intake in our sample. UIC/Cr (?g/g) was calculated by dividing UIC (?g/L) by creatinine concentration (g/L). Daily iodine intake was estimated using the following formula: UIC/Cr x predicted 24-hour creatinine excretion/0.92. Predicted 24-hour urine creatinine was estimated using an established equation for adult females as described in Krzeczkowski et al. (2022) (Krzeczkowski et al., 2022); 0.92 is the urinary iodine excretion rate.
2.5. Children’s intelligence
Intellectual abilities were assessed at 3-to-4 years of age using the Wechsler Preschool and Primary Scale of Intelligence-III [Canadian norms; mean (SD) = 100 (15)]. Testing was conducted in the child’s primary language (English or French) by trained research assistants who were blinded to women’s fluoride exposure and thyroid status in pregnancy. Integrity of test administration was ensured by periodic observation of testers and double scoring of all protocols. We used Full-Scale IQ (FSIQ), a measure of global intellectual and cognitive functioning, as the primary outcome. Verbal (VIQ) and Performance IQ (PIQ) were used in supplementary analyses.
2.6. Statistical analysis
We examined the distribution and descriptive statistics for all demographics, maternal fluoride exposure and thyroid hormone variables, and child FSIQ. We used Spearman correlations to examine associations between exposure variables. Our primary analyses used multinomial logistic regression to estimate odds of subclinical and primary hypothyroidism associated with each exposure measure (water fluoride concentration, fluoride intake, and MUFSG concentration). We adjusted for maternal age, level of education (dichotomized as bachelor’s degree or higher), pre-pregnancy body mass index (BMI), and race (White or Other). Race was self-reported by participants and dichotomized given the majority of women (86 %) in MIREC identified as White, with the remaining individuals identifying as other categories, including Asian/Pacific Islander, multiracial or other. Race was included as a covariate given evidence of disparities in women’s exposures to, and metabolism of, environmental chemicals (Nguyen et al., 2020), and evidence of differences in diagnosis of thyroid disorders by race (McLeod et al., 2014). Covariates were included in models based on prior studies suggesting that they are causal determinants of thyroid function and may be associated with fluoride exposure (Till et al., 2018; Buzalaf and Whitford, 2011; Collares et al., 2017). We also adjusted for study site when MUFSG was used as the independent variable because thyroid function problems could vary across the study sites. The same set of covariates was used in the models with fluoride intake and water fluoride concentration, apart from study site because site is collinear with fluoride levels in municipal drinking water (which was used to derive fluoride intake). Moreover, the models involving fluoride intake or water fluoride concentration only included women who reported drinking tap water in pregnancy. We tested for effect modification by maternal TPOAb status in all models through inclusion of interaction terms. If the interaction term was significant, we probed the predicted slopes by running each model twice, once with normal TPOAb levels (<5.61 IU/mL) set as the reference category, and again with high TPOAb levels (?5.61 IU/mL) as the reference.
For all models, odds ratios and associated confidence intervals (CIs) were reported per 0.5 mg/L or mg/day increase in water fluoride concentration, fluoride intake, and MUFSG concentration; 0.5 mg/L corresponds to the approximate difference in water fluoride concentration between a fluoridated and non-fluoridated community and interquartile range (IQR) for MUFSG, and 0.5 mg/day represents the approximate difference in dietary fluoride consumption between women at the 25th and 60th percentiles of fluoride intake. Regression diagnostics confirmed no assumption violations, issues with model fit, collinearity, heteroskedasticity, influential cases, or outliers in any of the above models.
As a secondary aim, we investigated the association between maternal hypothyroidism and child IQ. We used multiple linear regression to test whether subclinical or primary hypothyroidism was associated with child FSIQ; this analysis included a total of 466 mother-child dyads with thyroid and FSIQ data, of whom 411 women were classified as euthyroid, 27 women as subclinical hypothyroid, and 28 as primary hypothyroid. Effect modification by child sex was explored through inclusion of interaction terms. Given the smaller sample size, we probed an interaction if the p value for the interaction term was <0.15 (Rothman, 2014). We estimated slopes by running each model twice, once with female children set as the reference category, and again with male children as the reference. Models involving IQ were adjusted for maternal age, race, level of education, second-hand smoke exposure (yes/no/I don’t know), parity, study site, child sex, and a continuous measure of the quality of the home environment (HOME score). We did not adjust for gestational age in these models given that gestational age may be a causal intermediate between hypothyroidism in pregnancy and child IQ (Consortium on Thyroid and Pregnancy – Study Group on Preterm Birth, 2019; Nasirkandy et al., 2017); however, gestational age was added to our model in a sensitivity analysis. We further probed the association between primary hypothyroidism and IQ using VIQ and PIQ. Participants who were missing covariates (<7.5 % of total sample; see Fig. 1) were excluded from all models.

Fig. 1. Study sample flow chart for primary analyses.
We used STATA version 17.0 (STATA corporation) for all statistical analyses. Two-sided p values ?.05 were considered to indicate statistical significance.
2.7. Sensitivity analyses
For primary models investigating associations between fluoride exposure and hypothyroidism, we conducted the following sensitivity analyses: First, we entered thyroglobulin (Tg), a biomarker of long-term iodine nutrition, as a covariate in all three multinomial regression models used to estimate odds of subclinical or primary hypothyroidism associated with each exposure measure to evaluate confounding by iodine insufficiency in these relationships. Second, we ran three separate binary logistic regression models to estimate odds of primary hypothyroidism associated with water fluoride concentration, fluoride intake, and MUFSG concentration including only women who self-reported clinical diagnoses of primary hypothyroidism at the time of enrollment in MIREC. Third, we ran seven separate multinomial logistic regression models to estimate the association between water fluoride concentration and odds of primary hypothyroidism with adjustment for other environmental toxicants, including arsenic, lead, manganese, mercury, perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), and perfluorohexanesulfonic acid (PFHxS) measured in trimester one. Lastly, we reran the models used to estimate odds of either subclinical or primary hypothyroidism associated with maternal fluoride intake and MUFSG concentration using data from trimester one only given that these values were measured at the same time as the thyroid variables and may better represent pre-pregnancy fluoride exposure.
Given the association between hypothyroidism and child FSIQ, we explored whether primary hypothyroidism in pregnancy would mediate the significant association between water fluoride concentration and children’s FSIQ that we previously reported in the MIREC sample (Green et al., 2019). The current analysis was considered exploratory given the limited number of participants (n = 358) with data related to water fluoride concentration, child FSIQ, and thyroid status. Notably, only 25 of 107 (23.4 %) women in the primary hypothyroid group were retained in the mediation analysis. We used mediation analysis within the counterfactual framework (Valeri and VanderWeele, 2013) (via paramed package in STATA) to explore the potential mediating effect (or indirect effect) of primary hypothyroidism in the association between water fluoride concentration and child FSIQ. We estimated the effect of a 0.5 mg/L increase in water fluoride concentration on child FSIQ. Mediation models were rerun using child VIQ and PIQ.
3. Results
Fig. 1 shows the sample flow chart for all primary variables and subgroups of interest, with detailed descriptions of where there was missing exposure, outcome, and covariate data. We studied a total of 1508 women (mean (SD) age = 32.2 (5.02) years) with thyroid data, of whom 1301 women (86.3 %) were classified as euthyroid, 100 met criteria for subclinical hypothyroidism (6.6 %), and 107 (7.1 %) met criteria for primary hypothyroidism; among the primary hypothyroid group, 79 women reported a diagnosis at time of study enrollment and 28 were identified based on thyroid hormone levels measured in the first trimester. As expected, among the women who had TPOAb data, more women in the subclinical (33 of 100; 33.0 %) and primary hypothyroid (62 of 98; 63.3 %) groups had elevated TPOAb levels (?5.61 IU/mL) relative to the euthyroid group (137 of 1295; 10.6 %), suggesting active or incipient autoimmune hypothyroidism (Table 1). As predicted, water fluoride concentration was moderately-to-strongly associated with fluoride intake (r = 0.76, p < .01) and urinary fluoride (r = 0.49, p < .01) and fluoride intake was associated with urinary fluoride (r = 0.59, p < .01).
3.1. Maternal fluoride exposure and thyroid status
Among the 1105 women with a water fluoride measurement, 945 (85.5 %) were classified as euthyroid, 79 (7.2 %) as subclinical hypothyroid, and 81 (7.3 %) as primary hypothyroid. Mean water fluoride concentration was 0.42 mg/L (median = 0.52; range: 0.04 to 0.87 mg/L) and 60.5 % lived in fluoridated communities. Covariate-adjusted multinomial logistic regression revealed a statistically significant association between water fluoride concentration and risk of primary hypothyroidism; a 0.5 mg/L increase in water fluoride concentration was associated with 1.65 times greater odds (95 % CI: 1.04, 2.60) of having a diagnosis or meeting criteria for primary hypothyroidism. In contrast, no statistically significant association was observed between water fluoride concentration and risk of subclinical hypothyroidism (adjusted odds ratio [aOR]: 1.15; 95 % CI: 0.73, 1.82) (Fig. 2).

Fig. 2. Covariate-adjusted and unadjusted effect estimates of associations between water fluoride concentration, daily fluoride intake, and urinary fluoride concentration, and subclinical and primary hypothyroidism relative to euthyroid women.
Note. Covariate-unadjusted and adjusted effect estimates from multinomial logistic regression models shown in black and grey, respectively. Results are also summarized in Table 2.
OR = odds ratio; reported for every 0.5 mg/L or 0.5 mg/day increase in water fluoride concentration, MUFSG concentration, and daily fluoride intake.
Abbreviations: MUFSG = maternal urinary fluoride, standardized for specific gravity; CI = confidence interval
Of the 996 women with a fluoride intake measurement, 857 (86.1 %) were classified as euthyroid, 71 (7.1 %) as subclinical hypothyroid, and 68 (6.8 %) as primary hypothyroid. Mean fluoride intake was 0.67 mg/day (median = 0.58; range: 0.02 to 2.84 mg/day) and 61 % lived in fluoridated communities. Daily fluoride intake was not significantly associated with risk of subclinical (aOR: 1.03; 95 % CI: 0.81 to 1.32) or primary hypothyroidism (aOR: 1.25; 95 % CI: 0.99, 1.57) (Fig. 2).
Among the 1149 women with a SG-adjusted urinary-fluoride measurement, 991 (86.3 %) were classified as euthyroid, 83 (7.2 %) as subclinical hypothyroid, and 75 (6.5 %) as primary hypothyroid. Mean MUFSG was 0.59 mg/L (median = 0.49; range: 0.05 to 3.33 mg/L) and 59.5 % lived in fluoridated communities. Results from multinomial logistic regression analysis indicated that MUFSG concentration was not significantly associated with risk of subclinical hypothyroidism (aOR: 0.94; 95 % CI: 0.67, 1.31) or primary hypothyroidism (aOR: 1.00; 95 % CI: 0.73, 1.39) (Fig. 2). Results did not differ when using creatinine-adjusted MUF concentration (Table S3).
3.1.1. Effect modification by TPOAb status
The interaction between fluoride exposure and maternal TPOAb status in predicting risk of primary hypothyroidism was statistically significant for models with water fluoride concentration (p interaction term = 0.03) and daily fluoride intake (p interaction term = 0.01). Women with normal TPOAb levels were 2.85 times (95 % CI: 1.25, 6.50) more likely to have or meet criteria for primary hypothyroidism for each 0.5 mg/L increase in water fluoride concentration. Likewise, fluoride intake was significantly associated with risk of primary hypothyroidism among women with normal TPOAb levels (aOR: 1.75; 95 % CI: 1.27, 2.41). In contrast, there was no evidence of effect modification by maternal TPOAb status in the associations between water fluoride or fluoride intake and risk of subclinical hypothyroidism, or between MUFSG and risk of subclinical or primary hypothyroidism (Table 2).
3.2. Maternal hypothyroidism and child intelligence
Demographic characteristics comparing the water fluoride cohorts with and without child IQ data are summarized in Table S1. Compared to women without child IQ data, those with child IQ data were more likely to be White, live in non-fluoridated cities, report no second-smoke exposure in the first trimester, and had lower MUFSG and water fluoride concentrations, and daily fluoride intake.
Median (IQR) FSIQ score was 108 (19) for the sample of 439 children with euthyroid or primary hypothyroid mothers (females: 110 (17); males: 105 (19)). FSIQ scores were 4.45-points (95 % CI: ?9.17, 0.26) lower, on average, among children of primary hypothyroid women compared to children of euthyroid women. The interaction between maternal primary hypothyroidism and child sex in predicting child FSIQ scores met our threshold for model-selection purposes (p interaction term = 0.13). Males born to women with primary hypothyroidism (n = 13) had significantly lower FSIQ scores (B coefficient: ?8.42; 95 % CI: ?15.33 to ?1.50) compared with males born to euthyroid women (n = 201) (Fig. 3). In contrast, FSIQ scores did not differ significantly among females born to primary hypothyroid women (n = 15) versus euthyroid women (n = 210; B coefficient: ?1.04; 95 % CI: ?7.47, 5.38). Results remained the same with gestational age included as a covariate. Further probing of the association between maternal primary hypothyroidism and child IQ indicated that the significant difference in FSIQ among male children born to primary hypothyroid versus euthyroid women was primarily driven by a difference in VIQ (B coefficient: ?8.76; 95 % CI: ?15.59 to ?1.93) and not PIQ (B coefficient: ?5.93; 95 % CI: ?13.64 to 1.79; Fig. 3). For complete results, see Table S2.

Fig. 3. Sex-specific effects in the association between maternal primary hypothyroidism and child FSIQ, VIQ, and PIQ.
Note. Top (left to right): mean FSIQ, VIQ, and PIQ scores of male children born to euthyroid (mean: FSIQ = 105.06, VIQ = 107.3, PIQ = 101.97) and primary hypothyroid (mean: FSIQ = 95.92, VIQ = 99.1, PIQ = 94.23) women.
Bottom (left to right): mean FSIQ, VIQ, and PIQ scores of female children born to euthyroid (mean: FSIQ = 109.91, VIQ = 111.96, PIQ = 105.61) and primary hypothyroid (mean: FSIQ = 107.07, VIQ = 112.43, PIQ = 100.20) women.
Error bars represent the 95 % confidence intervals.
* Indicates a significant difference between means (p < .05).
We did not observe a significant association between maternal subclinical hypothyroidism and child FSIQ (n = 438; B coefficient: 0.05; 95 % CI: ?4.78, 4.89). Further, we found no evidence of effect modification by child sex.
3.3. Sensitivity analyses
3.3.1. Primary models
Controlling for Tg as a covariate in our models estimating associations between fluoride exposure (water fluoride concentration, fluoride intake, and MUFSG concentration) and risk of either subclinical or primary hypothyroidism did not alter any of the results (Table S3).
When the sample was restricted to include only women who reported clinical diagnoses of primary hypothyroidism at the time of enrollment in MIREC (n = 59), a 0.5 mg/L increase in water fluoride concentration was associated with 1.88 times greater odds (95 % CI: 1.10, 3.21) of having a diagnosis after controlling for covariates. Similarly, results revealed a statistically significant association between daily fluoride intake and diagnosed primary hypothyroidism (aOR: 1.42; 95 % CI: 1.10, 1.82). In contrast, no significant association was found between MUFSG concentration and diagnosed primary hypothyroidism (Table S3).
We found no evidence of confounding by other environmental toxicants (i.e., arsenic, lead, manganese, mercury, PFOA, PFOS, or PFHxS) in the association between water fluoride concentration and risk of primary hypothyroidism (Table S3).
Finally, MUFSG concentration from trimester one was not significantly associated with hypothyroidism (Table S3). In contrast, the association between daily fluoride intake and risk of primary hypothyroidism was statistically significant when using fluoride intake from trimester one in the model (aOR: 1.42; 95 % CI: 1.18, 1.71) (Table S3).
3.3.2. Mediation analysis
Mediation analysis on the subsample of 358 mother-child pairs with water fluoride, thyroid (i.e., euthyroid and primary hypothyroid women), and FSIQ data, as well as complete covariates, indicated a significant direct effect of water fluoride concentration on child FSIQ (natural direct effect estimate = ?3.55; 95 % CI: ?5.95, ?1.15). In contrast, the indirect effect was close to null (natural indirect effect estimate = ?0.12; 95 % CI: ?0.41, 0.16), indicating that maternal primary hypothyroidism did not significantly mediate the relationship between maternal water fluoride concentration and child FSIQ in this subsample of mother-child pairs (Table 3). Likewise, there was no evidence of mediation when the model was rerun using child VIQ and PIQ (Table S4).
Table 3. Maternal primary hypothyroidism as a mediator of the association between maternal water fluoride concentration and child FSIQ.
| Mediation parameters | B | p > |z| | 95 % Confidence Interval | |
|---|---|---|---|---|
| Natural direct effecta | ?3.55 | 0.00 | ?5.95 | ?1.15 |
| Natural indirect effectb | ?0.12 | 0.40 | ?0.41 | 0.16 |
| Marginal total effectc | ?3.67 | 0.79 | ?30.66 | 23.32 |
Output from mediation analysis in the counterfactual framework.
B = effect of a 0.5 mg/L increase in water fluoride concentration on child FSIQ.
- a
-
Effect of maternal water fluoride concentration on child FSIQ not mediated by maternal primary hypothyroidism.
- b
-
Effect of maternal water fluoride concentration on child FSIQ mediated by maternal primary hypothyroidism.
- c
-
Total effect of maternal water fluoride concentration on child FSIQ, mediated and not mediated by maternal primary hypothyroidism (i.e., sum of the natural directa and indirectb effects).
4. Discussion
In this Canadian pregnancy and birth cohort, fluoride in drinking water was associated with risk of primary hypothyroidism in pregnant women. Specifically, risk of hypothyroidism was 1.65 times higher per 0.5 mg/L increase in water fluoride concentration after accounting for potential confounding variables. To contextualize these results, the difference in water fluoride concentration between cities that are fluoridated at the recommended level of 0.7 mg/L and those without fluoridation is approximately 0.5 mg/L (Till et al., 2018). It is noteworthy that among the 1508 women for whom thyroid status was defined in the current study, 60.5 % lived in fluoridated areas, which is considerably higher than the proportion of the general Canadian population receiving fluoridated municipal drinking water (i.e., 38 %) (Public Health Agency of Canada, 2017). While fluoridated tap water is the main source of fluoride exposure among adults in fluoridated communities (United States Environmental Protection Agency, n.d.), we also estimated fluoride intake by weighting each cup of water and other water-based beverages (i.e., tea, coffee) consumed by fluoride concentration in tap water. Findings revealed the same pattern of results, where higher fluoride intake was associated with risk of primary hypothyroidism, particularly among women with normal TPOAb levels.
In contrast, maternal urinary fluoride concentration was not associated with hypothyroidism. Fluoride concentrations in municipal water supplies are relatively constant and therefore are more likely to be indicative of chronic fluoride exposure, and perhaps body burden, than urinary fluoride. It is possible that day-to-day variations in intake of high-fluoride foods, beverages, or dental products before sample collection could influence urinary-fluoride measurement and contribute to exposure misclassification that would bias estimates toward the null. Moreover, thyroid disorders tend to develop over time (Casey et al., 2017; Chatzitomaris et al., 2017). Thus, it is reasonable that our more stable measure of fluoride exposure (i.e., water fluoride concentration) would be more strongly associated with risk of primary hypothyroidism than maternal urinary fluoride. Among the women with primary hypothyroidism, 73.8 % reported having a diagnosis prior to their pregnancy and enrollment in the MIREC study. In contrast, urinary fluoride was measured throughout pregnancy, and thus, following diagnosis for most women. Therefore, our findings make sense temporally in that we would not expect an exposure variable measured in pregnancy to predict risk of a health condition diagnosed before pregnancy. Similarly, we found a stronger association between daily fluoride intake and risk of primary hypothyroidism when looking at fluoride intake in trimester one only, perhaps indicating that women’s self-reported beverage consumption in trimester one was more reflective of their pre-pregnancy beverage consumption habits. Prior research examining associations between fluoride exposure and other health outcomes have also reported stronger associations with water fluoride over urinary fluoride concentration (Green et al., 2019; Malin et al., 2018; Riddell et al., 2019).
We did not observe an association between maternal fluoride exposure and subclinical hypothyroidism. This may be because subclinical hypothyroidism is a milder form of hypothyroidism with more variable presentations that may be exacerbated by pregnancy-associated changes in thyroid hormone levels. Indeed, there is ongoing controversy as to whether subclinical hypothyroidism constitutes a clinical disorder that requires formal treatment, especially in pregnancy, given that abnormalities in thyroid hormone levels are commonly detected in routine blood work during pregnancy (Casey et al., 2017). Notably, when we restricted our analysis to include only women with a reported clinical diagnosis of hypothyroidism, the observed associations between maternal fluoride exposure and primary hypothyroidism were stronger than when we included women identified using trimester one thyroid hormone levels – perhaps reflecting imprecision in our classifications of maternal thyroid function.
Our findings are consistent with prior studies showing a relationship between fluoride exposure and thyroid function. An ecologic study conducted in England reported a significantly higher prevalence of diagnosed hypothyroidism among adults living in areas with higher fluoride levels in drinking water (Peckham et al., 2015). Considering hypothyroidism is defined by elevated TSH and low FT4 levels, our findings are also in line with epidemiologic studies reporting associations between higher drinking water-fluoride concentrations and higher TSH and lower T4 levels in children and adults (Khandare et al., 2018; Kheradpisheh et al., 2018). In contrast, our results do not align with those of a Canadian study that reported no relationship between fluoride exposure (measured in urine and tap water) and self-reported diagnosis of a thyroid disorder among non-pregnant adults (Barberio et al., 2017). Discrepancy in results could reflect differences in exclusion and inclusion criteria (i.e., the prior study included respondents between the ages of 3–79 years), differences between a pregnant and non-pregnant sample, and differences in how thyroid disorders were classified (i.e., use of different cut-offs for elevated TSH and low FT4).
Fluoride may impact thyroid function by several potential mechanisms. It may inhibit the deiodinase enzymes that are necessary for thyroid hormone production, resulting in decreased blood-T3 and T4 levels and increases in circulating TSH (Malin et al., 2018; Susheela et al., 2005). Fluoride may also induce structural and functional changes to the follicular epithelial cells of the thyroid gland (e.g., decline in the colloidal content and damage to the endoplasmic reticulum) resulting in insufficient secretion of Tg, and thus disruption to thyroid hormone synthesis more broadly (Banji et al., 2013; Basha et al., 2011). Further, fluoride may interfere with iodine to exert its negative effects on thyroid function, perhaps by inhibiting the expression and activity of sodium iodide symporters that are necessary for mediating active iodide transport into the thyroid, resulting in lower iodine availability and the indirect suppression of thyroid hormone production (Greer et al., 2002; Waugh, 2019). Importantly, however, a recent experimental study (Buckalew et al., 2020) refuted this claim by showing that fluoride does not inhibit sodium iodide symporter activity in Fischer rat thyroid follicular cells. This, together with our prior finding of women in MIREC being largely iodine sufficient (Krzeczkowski et al., 2022), and evidence that the effect of water fluoride concentration on maternal primary hypothyroidism remains significant after controlling for a biomarker of chronic iodine nutrition (i.e., Tg), suggest that iodine deficiency is not a confounder in this study. Ultimately, further research in this area is needed to understand fluoride action on thyroid function and whether iodine modifies the neurotoxicity of fluoride as reported in prior studies (Malin et al., 2018; Goodman et al., 2022).
In our study, having an underlying autoimmune condition (i.e., Hashimoto’s disease) did not increase vulnerability to fluoride-induced changes in thyroid gland functioning. Rather, our findings showed that pregnant women with normal TPOAb levels (<5.61 IU/mL) were most susceptible to fluoride-associated thyroid disruption, in that the associations between water fluoride concentration and fluoride intake and primary hypothyroidism were stronger among this group of women. It is possible that pregnant women with high TPOAb levels had a pre-existing autoimmune-related decreased capacity to produce thyroid hormones and therefore did not show as strong of a link with fluoride exposure compared to women with normal TPOAb levels. However, the estimates for women with normal TPOAb levels were less precise (i.e., wider confidence intervals) and should thus be interpreted with caution.
Our finding that women with primary hypothyroidism were more likely to have children with lower IQ scores is consistent with previous studies (Andersen et al., 2018; Haddow et al., 1999; Levie et al., 2018), though this association was only significant for FSIQ and VIQ among male children in our study. Few, if any, studies have explored effect modification by child sex when assessing the relationship between maternal hypothyroidism in pregnancy and offspring IQ. However, males born to women with hypothyroidism have been reported to be at increased risk of developing externalizing (e.g., attention deficit and hyperactivity disorder) (Peltier et al., 2021) problems when compared to females. In the context of neurotoxicants, the male brain is known to be more vulnerable to many chemical exposures, including fluoride, when compared with similarly exposed females (Green et al., 2020; Kern et al., 2017). Moreover, in recent studies, women who were pregnant with males were found to be more likely to have elevated TSH (Sitoris et al., 2022; Wang et al., 2019). Collectively, these findings suggest that sex differences in the association between maternal hypothyroidism in pregnancy and child FSIQ and VIQ are plausible.
Despite the significant direct effect between water fluoride concentration and lower child FSIQ and observed trend toward lower child FSIQ among women with primary hypothyroidism (p = .06), results from mediation analysis showed that maternal primary hypothyroidism did not significantly mediate the relationship between water fluoride concentration and child FSIQ. It is important to note that we were unable to account for all relevant covariates in the mediation model; study site was not included due to collinearity, and some of the covariates (e.g., HOME score) that are relevant for the association between hypothyroidism and child IQ are not directly relevant for the association between fluoride exposure and hypothyroidism. Moreover, considering only 25 of 107 (23.4 %) children of mothers with primary hypothyroidism had IQ data, there may have been insufficient statistical power in the mediation model to detect a significant indirect effect.
Among the sample of pregnant women included in this study, 6.1 % met criteria for primary hypothyroidism. Prevalence of primary or overt hypothyroidism has been shown to vary across other Canadian and American pregnant samples, generally falling between 0.7 and 3 % (Stagnaro-Green and Pearce, 2012; Leduc-Robert et al., 2020; Blatt et al., 2012). The relatively higher prevalence rate observed in the current study may be explained, in part, by the fact that women were categorized as primary hypothyroid if they met criteria based on their thyroid hormone levels in trimester one or if they had reported a previous diagnosis. Notably, women with prior diagnoses presented as euthyroid by blood panel in trimester one, and thus, would not normally be accounted for when determining prevalence rates based on thyroid hormone levels alone. This discrepancy may also be attributed to differences in diagnostic criteria used across studies (i.e., use of TSH on its own, with varying cut-offs) and time at thyroid hormone measurement since prevalence rates can vary across trimesters. Moreover, prevalence rates may differ depending on the demographic characteristics (e.g., race, age) of the sample (Stagnaro-Green and Pearce, 2012; Blatt et al., 2012).
4.1. Strengths and limitations
Strengths of our study include the use of multiple measures of maternal fluoride exposure and thyroid hormones measured using gold-standard approaches in a large pregnancy cohort. In addition, our analyses considered an array of potential confounding variables and incorporated several factors that may influence thyroid hormone levels, including Tg, TPOAb levels, and pre-pregnancy BMI. There are some limitations associated with our study as well. Women in the MIREC cohort tend to be older, more educated, more likely to be married or common law, primarily White, and more likely to report prenatal vitamin use (Arbuckle et al., 2013) which may restrict the generalizability of our results to the broader Canadian population. Results pertaining to child IQ may also be restricted in generalizability, given the subsample of mother-child dyads with available IQ data were more likely to reside in non-fluoridated areas (54.0 % of the fluoride-IQ sample lived in a non-fluoridated area compared with 38.1 % in the fluoride-thyroid sample; Table S1). Regarding our measure of maternal race, our prior work conducted in the MIREC pregnancy cohort (Till et al., 2018), as well as in another Canadian population (Riddell et al., 2021), did not find differences in urinary-fluoride levels by race. We therefore do not have evidence of disproportionate exposure to fluoride by race, as reported in a study conducted in U.S. children (Martinez-Mier and Soto-Rojas, 2010). Still, other factors that could differ by race, such as diet (Brooks et al., 2017) and urinary pH (Ekstrand et al., 1982), could contribute to differences in fluoride excretion and therefore control for race is warranted for increasing the accuracy of the estimates. Reporting bias is also possible in that some women may not have self-reported a previous diagnosis of a thyroid disorder or taking thyroid medication. Further, as with any observational study, we cannot exclude the potential for residual confounding, whereby unmeasured or imprecisely measured confounders prevent causal inferences from begin drawn from associations. Interpretation of findings should also consider the potential for reverse causality. We considered hypothyroidism as a potential mediator of the association between fluoride exposure and child IQ; however, it is conceivable that women with hypothyroidism may drink more water or other beverages given that hypothyroidism is associated with increased thirst. In this case, hypothyroidism would be associated with higher fluoride intake among women drinking fluoridated tap water and/or tea. The plausibility of reverse causality is unlikely, however, given that we would not expect more women with hypothyroidism to drink fluoridated over non-fluoridated water. An additional limitation is that fluoride was measured in spot samples instead of 24-hour urine samples or first morning voids, preventing us from being able to control for behaviours that could contribute to fluctuations in urinary fluoride concentration given the short half-life of fluoride (approximately 5 h). We attempted to mitigate the effects of this limitation by averaging urinary fluoride across all three trimesters. Finally, the questionnaire and methods used to estimate daily fluoride intake have not yet been validated; however, fluoride intake showed the expected associations with urinary fluoride and water fluoride concentrations, suggesting content validity of our derived variable.
4.2. Conclusions
To our knowledge, this is the first study to investigate the relationships between maternal fluoride exposure and thyroid function in a prospective pregnancy cohort receiving optimally fluoridated water. Our findings indicate that higher levels of fluoride exposure in pregnant women were associated with increased risk of hypothyroidism, supporting our hypothesis that fluoride exposure may disrupt thyroid function. Thyroid dysfunction in pregnancy may be one mechanism underlying the previously found association between fluoride exposure in pregnancy and offspring FSIQ in the MIREC cohort (Green et al., 2019), particularly among women with male children, though further research is warranted. Our findings are of public health significance given the large number of people exposed to fluoride in drinking water and the vital role of thyroid hormones in neurodevelopment.
CRediT authorship contribution statement
Meaghan Hall: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. Bruce Lanphear: Conceptualization, Funding acquisition, Investigation, Writing – review & editing. Jonathan Chevrier: Investigation, Methodology, Validation, Writing – review & editing. Rick Hornung: Methodology, Validation, Writing – review & editing. Rivka Green: Conceptualization, Methodology, Writing – review & editing. Carly Goodman: Methodology, Writing – review & editing. Pierre Ayotte: Funding acquisition, Investigation, Writing – review & editing. Esperanza Angeles Martinez-Mier: Investigation, Resources, Writing – review & editing. R. Thomas Zoeller: Investigation, Writing – review & editing. Christine Till: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Rick Hornung reports financial support was provided by National Institute of Environmental Health Sciences. Christine Till reports a relationship with Health Research Board, Dublin that includes: consulting or advisory. Meaghan Hall reports a relationship with NIEHS that includes: travel reimbursement. Christine Till reports a relationship with National Institute of Environmental Health Sciences that includes: travel reimbursement. Dr. Lanphear served as a non-retained expert witness in the federal fluoride case to describe the results of the fluoride studies using the MIREC cohort (Food & Water Watch, et al. vs. U.S. Environmental Protection Agency, United States District Court for the Northern District of California at San Francisco). He received no payment for his service.
Acknowledgments
The authors would like to extend a sincere thank you to Nicole Lupien, Stéphanie Bastien, and Romy-Leigh McMaster (Centre de Recherche, CHU Sainte-Justine), and the Maternal Infant Research on Environmental Chemicals (MIREC) Study Coordinating Staff for their administrative support, the MIREC site investigators, as well as the MIREC Biobank; Jillian Ashely-Martin for her review of our manuscript as the Knowledge Translation representative for the MIREC study; Alain LeBlanc from the Institut National de Santé Publique Québec (INSPQ) for free and total thyroxine measurement; Nathalie Ouellet at INSPQ; the team at the Institut Universitaire de Cardiologie et de Pneumologie de Québec (IUCPQ) for measuring thyroglobulin, thyroid stimulating hormone, and thyroid antibody levels; Christine Buckley, Frank Lippert, and Prithvi Chandrappa at the Indiana University School of Dentistry for their analysis of urinary fluoride; and Dr. Joanne Rovet for serving as a consultant to this work; Dr. John Krzeczkowski, Taylor McGuckin, Maddy Blazer, and Raichel Neufeld at York University for their valuable contributions to, and support of, this work.
This research was funded by the National Institute of Environmental Health Science [grant numbers R21ES027044, 2016–2019; R01ES030365, 2020–2025], and the Maternal-Infant Research on Environmental Chemicals Study was funded by the Chemicals Management Plan at Health Canada, the Ontario Ministry of the Environment, and the Canadian Institute for Health Research (CIHR) [grant number MOP-81285, 2006]. This work was also supported by a CIHR scholarship awarded to M.H.
*Original full-text article online at: https://www.sciencedirect.com/science/article/pii/S0048969722082523
