|
Return to Index
Page
ACTIVITY:
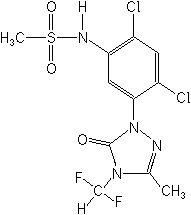
Herbicide (triazolone)
CAS Name:
N-[2,4-dichloro-5-[4-(difluoromethyl)-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazol-1-yl]phenyl]methanesulfonamide
Structure:
| |
| Published
Date |
Docket
Identification Number |
Details |
| August 25, 2006 |
EPA-HQ-OPP-2006-0659 |
Pesticide
Emergency Exemptions; Agency Decisions and State and Federal
Agency Crisis Declarations
• Iowa: Specific: EPA authorized
the use of sulfentrazone on strawberries
to
control broadleaf weeds; June 25, 2006 to December 15, 2006.
• Michigan: EPA authorized
the use of sulfentrazone on strawberries
to control
broadleaf weeds; June 25, 2006 to December 15, 2006.
• Wisconsin: Specific: EPA
authorized the use of sulfentrazone on strawberries
to control common groundsel; June 20, 2006 to December 15, 2006.
|
| June 7, 2006 |
EPA-HQ-OPP- 2006-0387 |
Pesticide
Emergency Exemptions; Agency Decisions and State and Federal Agency Crisis Declarations.
• North Dakota: Specific:
EPA authorized the use of sulfentrazone on flax
to control kochia; March 31, 2006 to June 30, 2006.
• Ohio: Specific: EPA authorized
the use of sulfentrazone on strawberries
to control common groundsel; June 20, 2006 to December 15, 2006.
• Oregon: Specific: EPA authorized
the use of sulfentrazone on strawberries
to control broadleaf weeds; March 15, 2006 to February 28, 2007.
• Washington: EPA authorized
the use of sulfentrazone on strawberries
to control broadleaf weeds; March 15, 2006 to February 28, 2007.
|
| August
31, 2005 |
OPP-2005-0223 |
Pesticide
Emergency Exemptions:
•
Michigan.
EPA authorized the use of sulfentrazone
on strawberries to control
broadleaf weeds; June 25, 2005 to December 15, 2005. Contact:
(Andrew Ertman). |
| August
3, 2005 |
OPP-2005-0201 |
Cancellation
of Pesticides for Non-payment of Year 2005 Registration Maintenance
Fees.
| Section
24(c) Registrations canceled for non-payment of the
2005
maintenance fee are shown in the following Table 1:
Table
1.--Section 24(c) Registrations Canceled for Non-Payment
of Maintenance Fee |
|
SLN no. |
Product
Name |
| 000279
ID-04-0001 |
Spartan
Herbicide |
| 000279
NV-04-0001 |
Spartan
Herbicide |
| 000279
OR-04-0024 |
Spartan
Herbicide |
| 000279
WA-04-0002 |
Spartan
Herbicide |
|
| July
13, 2005 |
OPP-2005-0188 |
Pesticide
Emergency Exemptions:
Oregon: EPA authorized the use
of sulfentrazone on strawberries
to control broadleaf weeds; March 11, 2005 to February 28, 2006.
Washington: EPA authorized the
use of sulfentrazone on strawberries
to control broadleaf weeds; March 2, 2005 to February 28, 2006.
Wisconsin: Specific: EPA authorized
the use of sulfentrazone on strawberries
to control broadleaf weeds; June 20, 2005 to December 15, 2005. |
| March
10, 2005 |
OPP-2005-0057 |
Requests
to Voluntarily Cancel Certain Pesticide Registrations.
Unless a request is withdrawn by September 6, 2005, orders will
be issued canceling these registrations. The Agency will consider
withdrawal requests postmarked no later than September 6, 2005.
| Chemical
Name |
Registration
No. |
Product
Name |
Company
Name and Address |
| Sulfentrazone |
000279
WA-04-0002 |
Spartan
Herbicide |
FMC
Corp. Agricultural Products
Group, 1735 Market St, Philadelph, PA 19103 |
|
| Feb
10, 2005 |
OPP-2005-0025 |
Removal
of Expired Time-limited Tolerances for Emergency Exemptions.
FINAL
RULE.
• 20.
Sulfentrazone. The time-limited tolerance
for chickpea seed is being
removed from Sec. 180.498 because
it expired on December 31, 2004. |
| Dec
10, 2004 |
OPP-2004-0392 |
Extension
of Tolerances for Emergency Exemptions. FINAL
RULE.
EPA has authorized under FIFRA section 18 the use of sulfentrazone
on lima beans and cowpeas for
control of hophornbeam copperleaf in
Tennessee. This regulation extends a time-limited tolerance
for combined residues of the herbicide
sulfentrazone and the metabolites 3-hydroxymethyl sulfentrazone
and 3-desmethyl sulfentrazone in
or on succulent bean seed without
pod at 0.1 ppm for an additional
3-year period. This tolerance will expire and is revoked on
December 31, 2007. A time-limited tolerance was originally
published in the Federal
Register of September 21, 1999 (64
FR 51060) (FRL-6097-8).
Note:
This FR of Sept 21, 1999, titled "Sulfentrazone; Pesticide
Tolerances for Emergency Exemptions" stated:
--
Developmental toxicity studies - Rats:
In EPA's oral developmental study in rats, the maternal
(systemic) NOAEL was 25 mg/ kg/day, based on increased relative
spleen weights and splenic extramedullary hematopoiesis
at the LOAEL of 50 mg/kg/day. The
developmental (fetal) NOAEL was 10 mg/kg/day, based on decreased
mean fetal weight and retardation in skeletal development
as evidenced by increased numbers of litters with any variation
and by decreased numbers of caudal vertebral and metacarpal
ossification sites at the LOEL of 25 mg/kg/day.
-- The developmental (fetal) NOAEL
was 100 mg/kg/day, based on decreased
fetal weight and increased fetal variations (hypoplastic
or wavy ribs, incompletely ossified lumbar vertebral arches,
incompletely ossified ischia or pubes, and reduced numbers
of thoracic vertebral and rib ossification sites) at the
LOAEL of 250 mg/kg/day.
-- Reproductive toxicity study --
Rats. In the 2-generation reproductive toxicity study
in rats- The reproductive NOAEL was 14/16 mg/kg/day, based
on: (a)
Increased duration of gestation in both F1 and F2 dams;
(b) decreased fertility in F1 generation
(males); and/or (c) atrophy
of the germinal epithelium of the testes, oligospermia and
intratubular degeneration of the seminal product in the
epididymis at the LOAEL of 33/40 mg/kg/day.
--
Chronic risk. RfD = 0.14 mg/kg/day.
For chronic dietary risk assessment
the Agency recommended use of the NOAEL of 14 mg/kg/day
with an uncertainty factor of 100, based on:
(a) Decreased maternal body
weight and/or body weight gain during gestation in
both P and F1 generations;
(b) reduced premating body
weight gains in the second generation
(F1 adults);
(c) increased duration of gestation
in both F1 and F2 dams;
(d) reduced prenatal viability
(fetal and litter);
(e) reduced litter size;
(f) increased number of stillborn
pups;
(g) reduced pup and litter
postnatal survival;
(h) decreased pup body weights
throughout lactation;
(i) decreased fertility in
F1 generation males; and
(j) atrophy of the germinal
epithelium of the testes, oligospermia and intratubular
degeneration of the seminal product in the epididymis at
the L
|
| Nov
10, 2004 |
OPP-2004-0232 |
Five
Pesticide Emergency Exemptions.
• Michigan - EPA authorized
the use of sulfentrazone on strawberries
to control broadleaf weeds; June 25, 2004 to December 15,
2004. Contact: (Andrew Ertman)
• Montana - On May 10,
2004, for the use of sulfentrazone
on flax to control kochia. This
program ended on June 30, 2004. Contact: (Andrew Ertman)
• Ohio
- EPA authorized the use of sulfentrazone
on strawberries to control groundsel;
June 3, 2004 to December 15, 2004. Contact: (Andrew Ertman)
• Tennessee
--- Crisis: On May 14, 2004,
for the use of sulfentrazone on cowpeas
to control Hophornbeam Copperleaf. This program is expected
to end on September 30, 2004. Contact: (Andrew Ertman)
--- Specific: EPA authorized
the use of sulfentrazone on cowpeas
to control Hophornbeam Copperleaf; May 14, 2004 to September
30, 2004. Contact: (Andrew Ertman) |
| May
24, 2004 |
OPP-2004-0136 |
Extension
of Tolerances for Emergency Exemptions. FINAL RULE.
EPA
has authorized under FIFRA section 18 the use of sulfentrazone
on flax for control of kochia and
ALS-resistant kochia in North
Dakota and South Dakota. This regulation extends
a time-limited tolerance for combined
residues of sulfentrazone and its metabolites 3-hydroxymethyl
sulfentrazone (HMS) and 3-desmethyl sulfentrazone (DMS) in or
on flax seed at 0.20 ppm for an additional 3-year
period. This tolerance will expire and is revoked on
December 31, 2007. A time-limited tolerance was originally published
in the Federal Register of August 21, 2002
|
| May
5, 2004 |
OPP-2004-0116 |
Pesticide
Emergency Exemptions.
North Dakota -
EPA authorized the use of sulfentrazone on flax
to control kochia; April 1, 2004 to June 30, 2004.
Oregon - EPA authorized the use
of sulfentrazone on strawberries
to control broadleaf weeds; March 26, 2004 to February 28,
2005.
South
Dakota
- EPA authorized the use of sulfentrazone on flax
to control ALS-resistant kochia; May 16, 2004 to June 30,
2004.
Washington - EPA authorized the
use of sulfentrazone on strawberries
to control broadleaf weeds; March
17, 2004 to February 28, 2005.
Wisconsin - EPA authorized the
use of sulfentrazone on strawberries
to control broadleaf weeds; June 20, 2004 to December
15, 2004. |
| Nov
26, 2003 |
OPP-2003-0358 |
Requests
for Pesticide Emergency Exemptions.
3 Denials:
-- Minnesota Department of Agriculture.
Denial. On June 13, 2003 EPA
denied the use of sulfentrazone on
potatoes to control nightshade. This request was denied
because yield losses were not supported by the submitted data.
-- North Dakota Department of Agriculture.
Denial. On June 13, 2003 EPA
denied the use of sulfentrazone on
potatoes to control nightshade. This request was denied
because yield losses were not supported by the submitted data.
-- Wisconsin Department of Agriculture.
Denial. On June 13, 2003, EPA
denied the use of sulfentrazone on
potatoes to control nightshade. This request was denied
because yield losses were not supported by the submitted data.
11 Approvals:
-- Idaho Department of Agriculture.
EPA authorized the use of sulfentrazone
on chickpeas to control
Russian thistle; May 13, 2003 to June 20, 2003.
-- Idaho Department of Agriculture.EPA
authorized the use of sulfentrazone
on potatoes to control ALS- inhibitor
and triazine resistant kochia, common lambsquarters and pigweed;
May 16, 2003 to June 15, 2003.
-- Illinois Department of Agriculture.
Specific. EPA authorized the use of sulfentrazone on horseradish
to control broad leaf weeds; April 15, 2003 to July 15, 2003.
-- Michigan Department of Agriculture.
EPA authorized the use of tetraconazole on sugarbeets
to control cercospora; June 6, 2003 to September 30, 2003.
-- Michigan Department of Agriculture.
EPA authorized the use of sulfentrazone on strawberries
to control broad leaf weeds; June 25, 2003 to December 15, 2003.
-- Ohio Department of Agriculture.
Specific. EPA authorized the use of sulfentrazone on strawberries
to control common groundsel; June 20, 2003 to December 15, 2003.
-- Oregon Department of Agriculture.
Specific. EPA authorized the use of sulfentrazone
on strawberries to control
broad leaf weeds; April 1, 2003 to February 28, 2004.
-- South Dakota Department of Agriculture.
Specific. EPA authorized the use of sulfentrazone on sunflowers
to control kochia; April 1, 2003 to June 30, 2003.
-- -- South Dakota Department of
Agriculture. EPA authorized the use of sulfentrazone
on flax to control kochia
and ALS-resistant kochia; May 16, 2003 to June 30, 2003.
-- Tennessee Department of Agriculture.
Specific. EPA authorized the use of sulfentrazone on
succulent beans to control hophornbeam copperleaf; May
15, 2003 to September 30, 2003.
-- Washington Department of Agriculture.
EPA authorized the use of sulfentrazone on chickpeas
to control Russian thistle; May 13, 2003 to June 20, 2003. |
| Sept
24, 2003
|
OPP-2003-0270
|
| FMC;
IR-4. Pesticide
Tolerances. FINAL RULE. Tolerances
are established for combined residues of the herbicide
sulfentrazone
and its metabolites HMS (N-(2,4-dichloro-5-(4-
(difluoromethyl)-4,5-dihydro-3-hydroxymethyl-5-oxo-1H-1,2,4-triazol-
1- yl)phenyl)methanesulfonamide) and DMS (N-(2,4-dichloro-5-(4-
(difluoromethyl)-4,5-dihydro-5-oxo-1H-1,2,4-triazol-1-
yl)phenyl)methanesulfonamide in or on the following food
commodities |
| Commodity |
Parts
per million |
Asparagus
Bean, lima, succulent
Corn, field, grain
Pea and bean, dried shelled, except soybean, subgroup
6C
Potato
Sugarcane, cane |
0.15 |
Cabbage
Corn, field, forage
Horseradish, roots
Peanut
Sugarcane, molasses
Sunflower, seed |
0.20 |
Corn,
field, stover
Peppermint, tops
Spearmint, tops |
0.30 |
| Peanut,
meal |
0.40 |
| Excerpts
from: Table 1.--Subchronic, Chronic, and Other Toxicity |
| Study
Type and Guideline No. |
Results |
| 90-Day
oral toxicity rodents (rats) - [870.3100] |
NOAEL
= 19.9 milligrams/kilogram/day (mg/ kg/day) for males
and 23.1 mg/kg/ day for females
LOAEL = 65.8 mg/kg/ day for males and 78.1 mg/kg/day for
females based on clinical signs of anemia
(reduced hematocrit, hemoglobin, mean cell volume, and
mean cell hemoglobin values during treatment) |
| 90-Day
oral toxicity rodents (mice) -
[870.3100] |
NOAEL
= 60 mg/kg/day for males and 79.8 mg/kg/day for females
LOAEL = 108.4 mg/ kg/day for males and 143.6 mg/kg/ day
for females based on decreased body
weights, body weight gains, red blood cells, hemoglobin,
hematocrit, and severity of splenic micropathology (increased
incidence and severity of extramedullary hematopoiesis) |
| 90-Day
oral toxicity in nonrodents (dogs)
- [870.3150] |
NOAEL
= 28 mg/kg/day
LOAEL = 57 mg/kg/ day for males and 73 mg/kg/day for females
based on decreased body weights
(7-10%) and body weight gains during first 5 weeks of
study; decreased hemoglobin, hematocrit, mean cell volume,
mean cell hemoglobin and mean cell hemoglobin concentration,
and increased absolute liver weights and alkaline phosphatase
levels, and microscopic changes in the liver and spleen
(pigmented sinusoidal microphages in the liver, swollen
centrilobular hepatocytes and pigmented reticuloendotheli
al cells in the spleen) |
| Prenatal
developmental in rodents (rats)
- [870.3700] |
Maternal
NOAEL = 25 mg/kg/day
LOAEL = 50 mg/kg/ day based on increased
relative splenic extramedullary hematopoiesis
Developmental NOAEL = 10 mg/kg/ day
LOAEL = 25 mg/kg/ day based on decreased
mean fetal weights, and retardation in skeletal development
evidenced by an increased number of litters with any
variation and by decreased number of caudal vertebral
and metacarpal ossification sites
Maternal
NOAEL = 250 mg/kg/day
LOAEL was not established.
Developmental NOAEL = 100 mg/kg/ day
LOAEL = 250 mg/kg/ day based on decreased
fetal body weight; increased incidence of fetal variations:
hypoplastic or wavy ribs, incompletely ossified lumbar
vertebral arches, and incompletely ossified ischia or
pubis; and reduced number of thoracic vertebral and
rib ossification sites |
| Prenatal
developmental in nonrodents (rabbits)
- [870.3700] |
MaternalNOAEL
= 100 mg/kg/ day
LOAEL = 250 mg/kg/ day based on increased
abortions, clinical signs (hematuria and decreased feces),
and reduced body weight gain
Developmental NOAEL = 100 mg/kg/ day
LOAEL = 250 mg/kg/ day based on increased
resorptions, decreased live fetuses per litter, and decreased
fetal weights |
|
2-Generation reproduction and fertility effects (rats)
- [870.3800] |
Parental/Systemic
NOAEL = 14 mg/kg/day for males and 16 mg/kg/day for females
LOAEL = 33 mg/kg/ day for males and 40 mg/kg/day for females
based on decreased maternal body weight/body weight gain
during gestation in both generation (P and F1) and reduced
premating body weight gain in second generation (F1) males
Reproductive NOAEL = 14 mg/kg/ day for males and 16 mg/kg/day
for females
LOAEL = 33 mg/kg/ day for males and 40 mg/kg/day for females
based on increased duration of gestation
in females and degeneration and/ or atrophy of the germinal
epithelium of the testes and oligospermia and intratubular
degenerated seminal material in the epididymis of F1 males
Offspring NOAEL = 14 mg/kg/ day for males and 16 mg/kg/day
for females
LOAEL = 33 mg/kg/ day for males and 40 mg/kg/day for females
based on reduced prenatal viability
(fetal and litter), reduced litter size, increased number
of stillborn pups, reduced pup and litter postnatal survival
and decreased pup body weights throughout lactation
|
| Reproduction
and fertility effects (rat) Nonguideline
- [870.3800] |
Parental/Systemic
NOAEL = 20 mg/kg/day
LOAEL = 51 mg/kg/ day (F1 females) based on decrease
in pre-mating body weight gain (10%)
Offspring and Reproductive NOAEL = 16 mg/kg/ day
LOAEL = 40 mg/kg/ day based on reduced
gestation day 20 fetal weights; decreased postnatal day
0, 4 and 7 pup weights; decreased pup survival; delayed
vaginal patency; reduced epididymal, prostate, and testicular
weights Additional information supports the conclusions
reached in the 2- generation reproduction study in rats |
| Chronic
toxicity dogs - [870.4100] |
NOAEL
= 24.9 mg/kg/day for males and 29.6 mg/kg/day for females
LOAEL = 61.2 mg/kg/ day for males and 61.9 mg/kg/day for
females based on compensated normochromic
microcytosis |
|
Carcinogenicity mice - [870.4200] |
NOAEL
= 93.9 mg/kg/ day for males and 116.9 mg/kg/day for females
LOAEL = 160.5 mg/ kg/day for males and 198.0 mg/kg/ day
for females based on dose- related decreases
in hemoglobin and hematocrit by study termination
No evidence of carcinogenicity |
|
Combined chronic toxicity/carcinogenicity rats
- [870.4300] |
NOAEL
= 40 mg/kg/day for males and 36.4 mg/kg/day in females
LOAEL = 82.2 mg/kg/ day for males and 67 mg/kg/day for
females based on dose-related decreased
body weights (11 and 19%), body weight gains (13 and 26%),
food consumption (13 and 19%), hemoglobin, hematocrit,
mean cell volume, and mean cell hemoglobin. Increased
nucleated red blood cells and reticulocytes in bone of
females at 124.7 mg/kg/ day No evidence of carcinogenicity |
|
Subchronic neurotoxicity screening battery - [870.6200] |
NOAEL
= 30 mg/kg/day for males and 37 mg/kg/day for females
LOAEL = 150 mg/kg/ day for males and 180 mg/kg/day for
females based on increased incidence
of clinical signs; decreased body weight, body weight
gains, and food consumption in females; and increased
motor activity in females. At 5,000 ppm, included
increased mortality; decreased
body weights, and body weight gains in males; decreased
hindlimb grip strength and increased tail flick latency
in males at week 8; distended bladders with red fluid
and enlarged spleen. No evidence of neuropathology
at 2,500 and 5,000 ppm. |
|
| May
7, 2003 |
OPP-2003-0149 |
Emergency
Exemptions for pesticide use.
Colorado
Department of Agriculture. EPA authorized the use of sulfentrazone
on potatoes to control
ALS- inhibitor and triazine-resistant kochia; March 2, 2003
to June 15, 2003.
Colorado
Department of Agriculture. EPA authorized the use of
sulfentrazone on sunflowers
to control broadleaf weeds; April 1, 2003 to July 1, 2003.
Kansas
Department of Agriculture. EPA authorized the use of sulfentrazone
on sunflowers to control kochia;
April 15, 2003 to July 1, 2003.
Minnesota
Department
of Agriculture. EPA authorized the use
of sulfentrazone on horseradish
to control broadleaf weeds; April 1, 2003 to July 1, 2003.
Minnesota
Department of Agriculture. EPA authorized the use of sulfentrazone
on sunflowers to control kochia;
April 15, 2003 to June 30, 2003.
Missouri
Department of Agriculture EPA authorized the use of sulfentrazone
on sunflowers to control broadleaf
weeds; April 1, 2003 to July 31, 2003.
Montana
Department of Agriculture. Specific: EPA authorized the use
of sulfentrazone on chick peas
to control wild buckwheat; March 13, 2003 to June 30, 2003.
Montana
Department of Agriculture. EPA authorized the use of sulfentrazone
on dry peas to control wild buckwheat;
March 13, 2003 to June 30, 2003.
Montana
Department of Agriculture. EPA authorized the use of sulfentrazone
on sunflowers to control kochia;
March 15, 2003 to June 30, 2003.
Nebraska
Department of Agriculture EPA authorized the use of sulfentrazone
on chickpeas to control broadleaf
weeds; March 27, 2003 to July 1, 2003.
Nebraska
Department of Agriculture. EPA authorized the use of sulfentrazone
on sunflowers to control broadleaf
weeds; April 1, 2003 to July 1, 2003.
Nebraska
Department of Agriculture. EPA authorized the use of sulfentrazone
on potatoes to control ALS- inhibitor
and triazine-resistant Palmer amaranth, redroot pigweed, and
common waterhemp; April 10, 2003 to June 30, 2003.
North
Dakota
Department of Agriculture. EPA authorized the use of sulfentrazone
on flax to control kochia and
ALS-resistant kochia; April 1, 2003 to June 30, 2003.
North
Dakota Department of Agriculture. EPA authorized the
use of sulfentrazone on chick peas to control wild buckwheat;
April 1, 2003 to June 30, 2003.
North
Dakota Department of Agriculture. EPA authorized the
use of sulfentrazone on field peas
to control wild buckwheat; April 1, 2003 to June 30, 2003.
North
Dakota Department of Agriculture. EPA authorized the
use of sulfentrazone on sunflowers to
control kochia; April 15, 2003 to June 30, 2003.
Oklahoma
Department of Agriculture. EPA authorized the use of sulfentrazone
on sunflowers to control broadleaf
weeds; April 15, 2003 to July 15, 2003.
South
Dakota
Department of Agriculture. EPA authorized the use of sulfentrazone
on chick peas to control kochia;
April 1, 2003 to June 30, 2003.
South
Dakota Department
of Agriculture. EPA authorized the use of sulfentrazone on
dry peas to control kochia; April 1, 2003 to June 30,
2003.
Texas
Department of Agriculture. EPA authorized the use of
sulfentrazone on sunflowers to
control broadleaf weeds; March 20, 2003 to June 30, 2003.
Washington
Department of Agriculture. EPA authorized the use of sulfentrazone
on strawberries to control broadleaf
weeds; March 15, 2003 to February 28, 2004.
Wisconsin
Department of Agriculture, Trade, and Consumer Protection.
EPA authorized the use of sulfentrazone on horseradish
to control broadleaf weeds; April 15, 2003 to July
15, 2003.
Wisconsin
Department
of Agriculture, Trade, and Consumer Protection. EPA authorized
the use of sulfentrazone on strawberries
to control common groundsel; June 20, 2003 to December 15,
2003.
Wyoming
Department of Agriculture. EPA authorized the use of sulfentrazone
on sunflowers to control
broadleaf weeds; April 15, 2003 to June 30, 2003. |
| March
7, 2003 |
OPP-2003-0011.
|
IR-4
and FMC.
Seven Pesticide
Petitions:
IR-4:
0E6149 proposes the establishment of a tolerance for sunflower,
seed at 0.2 ppm;
IR-4:
1E6311 proposes the establishment of
tolerances for horseradish, roots at 0.2 ppm, cabbage
at 0.2 ppm, peppermint, tops at 0.3 ppm, and spearmint, tops
at 0.3 ppm.
IR-4: 2E6405
proposes the establishment of a tolerance for potato
at 0.1 ppm.
IR-4: 2E6498
proposes the establishment of a tolerance for bean,
lima, succulent at 0.15 ppm.
IR-4: 2E6500
proposes the establishment of a tolerance for asparagus
at 0.15 ppm.
FMC: 0F6116
proposes the establishment of tolerances for peanut
nutmeat and its processed parts at 0.2 ppm, and sugarcane
and its processed parts at 0.1 ppm.
FMC: 2F6391
proposes the establishment of tolerances for corn,
field, forage at 0.25 ppm, corn, field, stover at 0.35 ppm;
pea and bean, dried shelled, except soybean, subgroup 6C at
0.15 ppm.
-- Genotoxicity... A mouse lymphoma
forward gene mutation assay yielded negative results with
equivocal results without activation.
-- Reproductive and developmental toxicity.
Significant treatment-related increases in the fetal
and litter incidences of incompletely ossified lumbar vertebral
arches, hypoplastic or wavy ribs, and incompletely ossified
or nonossified ischia or pubes occurred
at the high-dose (250 mg/kg/day). An additional significant
increase in the high-dose fetal incidence of variations
in the sternebrae (incompletely ossified or unossified) was
not judged to be treatment-related. At
250 mg/kg/day, the mean numbers of thoracic vertebral
and rib ossification sites were significantly decreased, a
high-dose effect of treatment with sulfentrazone consistent
with the significant treatment-related hypoplasia observed
in the skeletal evaluation of the ribs.
-- A developmental toxicity study in
rabbits... Skeletal evaluation in fetuses revealed dose-related
and treatment-related findings at the 375 mg/kg/day dose level.
These included significant increases in both the fetal and
litter incidences of fused caudal vertebrae (a malformation)
and of partially fused nasal bones (a variation). In addition,
at 375 mg/kg/day, significant treatment- related reductions
in ossification site averages were observed for metacarpals
and both forepaw and hindpaw phalanges.
-- A 2-generation reproduction study in the rat at dietary
levels of 14, 33, or 46 mg/kg/day in males and 16, 40, or
56 mg/kg/day in females established a NOAEL for systemic and
reproductive/developmental parameters of 14 mg/kg/day for
males and 16 mg/kg/day for females... Systemic effects were
comprised of decreased body weight gains, while reproductive/
developmental effect at the LOAEL included degeneration and/or
atrophy in the testes, with epididymal sperm deficits, in
the second (F1) generation males. Male fertility in the F1
generation was reduced at higher doses; litter size, pup survival,
and pup body weight for both generations were also effected
at higher doses.
-- Subchronic toxicity. A 90-day subchronic toxicity study
was conducted in rats, with dietary intake levels of 0, 3.3,
6.7, 19.9, 65.8, 199.3, or 534.9 mg/kg/day for males and 0,
4, 7.7, 23.1, 78.1, 230.5, or 404.3 mg/kg/day for females
respectively. NOAELs of 19.9 mg/ kg/day in males and 23.1
mg/kg/day in females were based on clinical anemia.
-- A 90-day subchronic feeding study was conducted in mice
by dietary admix at doses of 0, 10.3, 17.8, 60.0, 108.4, or
194.4 mg/kg/day for males and 0, 13.9, 29.0, 79.8, 143.6,
or 257.0 mg/kg/day for females, respectively. NOAELs of 60
mg/kg/day (males) and 79.8 mg/kg/day (females) were based
on decreases in body weights and/or gains; decreased erythrocytes,
hemoglobin (Hgb) and hematocrit (HCT) values; and splenic
microscopic pathology.
-- In a 90-day subchronic feeding study in dogs administered
by dietary admix at doses of 0, 10, 28, or 57 mg/kg/day for
males and 0, 10, 28, or 73 mg/kg/day for females, a NOAEL
of 28 mg/kg/day was determined for both males and females
based on decreases in Hgb and HCT, elevated alkaline phosphatase
levels, increased liver weights and microscopic liver as well
as splenic changes.
-- Chronic toxicity. A 12-month feeding study in dogs was
dosed at levels of 0.0, 24.9, or 61.2 mg/kg/day for male dogs
and 0.0, 10.4, 29.6, or 61.9 [[Page 11100]] mg/kg/day for
female dogs in the control through high-dose groups, respectively,
with a NOAEL of 24.9 mg/kg/day for males and 29.6 mg/kg/ day
for females based on hematology effects and microscopic liver
changes.
-- In a 24-month chronic feeding/carcinogenicity study in
rats at dietary doses of 0, 24.3, 40.0, 82.8, or 123.5 mg/kg/day
for males and 20.0, 36.4, 67.0, or 124.7 mg/kg/day for females,
an overall NOAEL of 40.0 mg/kg/day in males and 36.4 mg/kg/day
in females was based on hematology effects and reduced body
weights. There was no evidence of a carcinogenic response.
|
| Feb
24, 2003 |
OPP-2003-0033 |
Pesticide
Emergency Exemptions.
Michigan.
Specific: EPA authorized the use of sulfentrazone
on strawberries to control
broadleaf weeds; October 21, 2002 to December 15, 2002. |
| Jan
16, 2003 |
OPP-2002-0336
|
Extension
of Tolerances for Emergency Exemptions. EPA has authorized
under FIFRA section 18 the use of sulfentrazone on
horseradish for control of weeds in Wisconsin, Minnesota,
and Illinois; sugarcane for control of weeds in Louisiana;
and sunflowers for control of weeds in Montana, North Dakota,
Minnesota, Wyoming, Texas, Nebraska, Oklahoma, Missouri, South
Dakota, Kansas, and Colorado. This regulation extends
a time-limited tolerance for combined residues of the herbicide
sulfentrazone, N-[2,4-dichloro- 5-[4-(difluoromethyl)-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazol-1-
yl]phenyl]methanesulfonamide, and its metabolites 3-hydroxymethyl
sulfentrazone (HMS) and 3-desmethyl sulfentrazone (DMS) in
or on horseradish, roots at 0.1 ppm, sugarcane at 0.05 ppm,
and sunflower at 0.1 ppm for an additional 3-year period.
These tolerances will expire and are revoked on December
31, 2005. A time-limited tolerance was originally published
for sunflowers in the Federal Register of September 21, 1999
(64 FR 51060) (FRL-6097-8). Time-limited tolerances were originally
published for horseradish and sugarcane in the Federal Register
of November 9, 2000 (65 FR 67272) (FRL-6751-7). |
| Nov
27, 2002 |
OPP-2002-0313 |
EPA
approved the use of Sulfentrazone for 5 Emergency Exemption.
--
Colorado: Crisis: On June 4, 2002, for
the use of sulfentrazone on potatoes
to control broadleaf weeds. This program ended on July 1,
2002.
-- Louisiana: On May 6, 2002,
for the use of sulfentrazone on sugarcane
to control morning glories. This program is expected to end
on December 31, 2002.
-- Louisiana: Specific: EPA authorized
the use of sulfentrazone on sugarcane
to control morning glories; May 6, 2002 to December 31, 2002.
-- Nebraska: Crisis: On May 21,
2002, for the use of sulfentrazone on potatoes
to control broadleaf weeds. This program ended on July 1,
2002.
-- Pennsylvania: Specific: EPA
authorized the use of sulfentrazone on strawberries
to control common groundsel; June 26, 2002 to December 15,
2002. |
| August
7, 2002 |
OPP-2002-0164 |
Emergency
Exemptions for pesticide use.
Colorado
Department of Agriculture - EPA authorized the use of sulfentrazone
on chickpeas to control broadleaf
weeds; April 24, 2002 to June 30, 2002.
Illinois
Department of Agriculture - EPA authorized the use of sulfentrazone
on horseradish to control broadleaf weeds; April 15,
2002 to July 15, 2002.
Minnesota
Department of Agriculture - EPA authorized the use of sulfentrazone
on horseradish to control broadleaf weeds; April 15,
2002 to July 15, 2002.
Montana
Department of Agriculture - EPA authorized the use of sulfentrazone
on chickpeas and dried peas to control kochia; April
9, 2002 to June 30, 2002.
Nebraska
Department
of Agriculture - EPA authorized the use of sulfentrazone on
chickpeas to control broadleaf weeds; April 12, 2002
to July 1, 2002.
Nebraska
Department of Agriculture - EPA authorized the use of sulfentrazone
on potatoes to control broadleaf
weeds; May 21, 2002 to July 1, 2002.
North
Dakota Department
of Agriculture - EPA authorized the use of sulfentrazone on
flax to control kochia and ALS-resistant kochia; April
1, 2002 to June 30, 2002.
South
Dakota
Department of Agriculture - Specific: EPA authorized the use
of sulfentrazone on chickpeas and dried
peas to control kochia; April 9, 2002 to June 30, 2002.
Tennessee
Department of Agriculture - Specific: EPA authorized the use
of sulfentrazone on lima beans and cowpeas
to control hophornbeam copperleaf; May 30, 2002 to September
30, 2003.
Wisconsin
Department
of Agriculture, Trade, and Consumer Protection - EPA authorized
the use of sulfentrazone on horseradish
to control broadleaf weeds; April 15, 2002 to July 15, 2002.
Wisconsin
Department of Agriculture, Trade, and Consumer Protection
- EPA authorized the use of sulfentrazone on
strawberries to control common groundsel; June 20,
2002 to December 15, 2002. |
| July
17, 2002 |
OPP-2002-0112
|
Extension
of Tolerances for Emergency Exemptions. FINAL RULE.
EPA has authorized under FIFRA section 18 the use of sulfentrazone
on lima beans and cowpeas for control
of Hophornbeam Copperleaf in Tennessee. This regulation extends
a time- limited tolerance for combined
residues of the herbicide sulfentrazone and the metabolites
3-hydroxymethyl sulfentrazone and 3-desmethyl sulfentrazone
in or on succulent bean seed without pod at 0.1 ppm for an additional
2-year period. This tolerance will expire
and is revoked on December 31, 2004. The time-limited
tolerance was originally published in the Federal Register of
September 21, 1999 (64 FR 51060) (FRL-6097-8) (40 CFR 180.498).
|
| Nov
14, 2001 |
OPP-181082 |
Pesticide
Emergency Exemptions. EPA authorized the use in:
Colorado:
on sunflowers to control broadleaf
weeds; March 16, 2001 to July 1, 2001.
Kansas:
on sunflowers to control kochia;
March 15, 2001 to June 15, 2001.
Louisiana:
on sugarcane to control morning
glory; March 14, 2001 to Dec 31, 2001.
Minnesota:
on sunflowers to control kochia;
March 15, 2001 to June 30, 2001.
Missouri:
on sunflowers to control broadleaf
weeds; March 28, 2001 to July 31, 2001.
Montana:
Crisis: On April 26, 2001, for the use on chickpeas
and dried peas to control wild buckwheat. This program
ended on June 30, 2001.
Montana:
on sunflowers to control kochia;
March 15, 2001 to June 30, 2001.
Nebraska:
on sunflowers to control broadleaf
weeds; March 16, 2001 to July 1, 2001.
North
Dakota:
on chickpeas and dry peas to
control wild buckwheat. This program ended on June 30, 2001.
North
Dakota:
on sunflowers to control kochia;
March 15, 2001 to June 30, 2001.
North
Dakota:
on chick peas and dry peas to control wild buckwheat; April
24, 2001 to June 30, 2001.
Ohio:
on strawberries to control common
groundsel; June 20, 2001 to December 15, 2001.
Oklahoma:
on sunflowers to control broadleaf
weeds; May 3, 2001 to July 15, 2001.
South
Dakota:
Crisis: On April 19, 2001, for the use on chickpeas
and dried peas to control kochia. This program ended
on June 30, 2001.
South
Dakota:
on sunflowers to control kochia;
March 16, 2001 to June 30, 2001.
Tennessee:
on lima bean to control hophornbeam
copperleaf; April 16, 2001 to September 30, 2001.
Tennessee:
on cowpea to control hophornbeam
copperleaf; April 16, 2001 to September 30, 2001.
Texas:
on sunflowers to control broadleaf
weeds; March 28, 2001 to June 30, 2001.
Wyoming:
on sulflowers to control broadleaf
weeds; March 30, 2001 to July 1, 2001 |
| Aug
1, 2001 |
OPP-301154 |
Pesticide
Tolerances for Emergency Exemptions. - FINAL RULE.
Chickpea, seed at 0.10 ppm; Pea, dry, seed at 0.10 ppm; and
Strawberry at 0.6 ppm. Expiration date: December 31, 2004. Docket
control number OPP-301154 |
| Nov
9, 2000 |
OPP-301074 |
Pesticide
Tolerances for Emergency Exemptions. - FINAL RULE. |
| Sept
21, 1999 |
OPP-300903 |
Pesticide
Tolerances for Emergency Exemptions.. - FINAL RULE.
-- Developmental toxicity studies
- Rats: In EPA's oral developmental study in rats, the maternal
(systemic) NOAEL was 25 mg/ kg/day, based on increased relative
spleen weights and splenic extramedullary hematopoiesis at the
LOAEL of 50 mg/kg/day. The developmental
(fetal) NOAEL was 10 mg/kg/day, based on decreased mean fetal
weight and retardation in skeletal development as evidenced
by increased numbers of litters with any variation and by decreased
numbers of caudal vertebral and metacarpal ossification sites
at the LOEL of 25 mg/kg/day.
-- The developmental (fetal) NOAEL was 100 mg/kg/day, based
on decreased fetal weight and increased
fetal variations (hypoplastic or wavy ribs, incompletely ossified
lumbar vertebral arches, incompletely ossified ischia or pubes,
and reduced numbers of thoracic vertebral and rib ossification
sites) at the LOAEL of 250 mg/kg/day.
-- Reproductive toxicity study -- Rats. In the 2-generation
reproductive toxicity study in rats- The reproductive NOAEL
was 14/16 mg/kg/day, based on: (a) Increased duration of gestation
in both F1 and F2 dams; (b) decreased
fertility in F1 generation (males); and/or (c) atrophy
of the germinal epithelium of the testes, oligospermia and intratubular
degeneration of the seminal product in the epididymis at the
LOAEL of 33/40 mg/kg/day.
--
Chronic risk. RfD = 0.14 mg/kg/day.
For chronic dietary risk assessment the
Agency recommended use of the NOAEL of 14 mg/kg/day with an
uncertainty factor of 100, based on: (a) Decreased maternal
body weight and/or body weight gain during gestation in both
P and F1 generations; (b) reduced premating body weight gains
in the second generation (F1 adults); (c) increased duration
of gestation in both F1 and F2 dams; (d) reduced prenatal viability
(fetal and litter); (e) reduced litter size; (f) increased number
of stillborn pups; (g) reduced pup and litter postnatal survival;
(h) decreased pup body weights throughout lactation; (i) decreased
fertility in F1 generation males; and (j) atrophy of the germinal
epithelium of the testes, oligospermia and intratubular degeneration
of the seminal product in the epididymis at the LOAEL of 33/44
mg/kg/day for males and females, respectively, from a 2-generation
reproductive toxicity study in rats.
Docket
control number [OPP-300903] |
| May
1, 1998 |
na |
Amendments
to Tolerances. Correction of Effective Date. - FINAL
RULE. |
| Sept
27, 1995 |
na |
FMC
- Establishment
of a Temporary Tolerance of 0.05 ppm for Soybeans. |
| April
16, 1997 |
na |
FMC
- Conditional
approval of 5 Pesticide Product Registrations.
The applications were approved on February 27, 1997, for
1. Sulfentrazone Technical for manufacturing use only (EPA
Registration No. 279-3149).
2. Authority 4F (formerly Sulfentrazone 4F) for preemergence
and preplant incorporated weed control in soybeans (EPA Registration
No. 279-3146).
3. Authority 75DF (formerly Sulfentrazone 75DF for preemergence
and preplant incorporated weed control in soybeans (EPA Registration
No. 279-3148)
4. Authority BL for use on soybeans in preemergency, preplant
incorporated, no-till, and minimum till applications (EPA
Registration No. 279-3175)
5. Authority Broadleaf for use on soybeans in preemergency,
preplant incorporated, no-till, and minimum till applications
(EPA Registration No. 279-3179). |
| Mar
10, 1997 |
PF-670/OPP-300459 |
Establishment
of Tolerances. - FINAL RULE. |
| Nov
6, 1996 |
PF-670 |
FMC
- Pesticide
Tolerance Petition for residues in or on Soybeans. |
| Oct
5, 1995 |
na |
FMC
- Extension
of Experimental Use Permits. |
| March
8 1995 |
na |
FMC
- Application
to register 3 pesticide products. |
|