|
Return to Index
Page
Adverse
Effects
ACTIVITY:
Herbicide
(Sulfonylurea)
CAS Name:
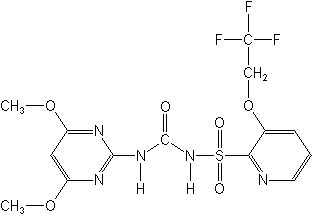
N-[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]-3-(2,2,2-trifluoroethoxy)-2-pyridinesulfonamide
Structure
for Trifloxysulfuron:
| |
| Date
Published |
Docket
Identification Number |
Details |
| April 13, 2007 |
EPA-HQ-OPP-2007-0005 |
Notice
of Receipt of Requests to Voluntarily Cancel Certain Pesticide
Registrations.
| Registration No. |
Product Name |
Registrant |
| 000100 TX-06-0019 |
Envoke Herbicide |
Syngenta Crop
Protection, Inc.
Po Box 18300
Greensboro NC 274198300 |
|
| Sept
17, 2003 |
OPP-2003-0286 |
| SYNGENTA:
Pesticide
tolerances. FINAL RULE. |
| Commodity |
Final
Tolerance
PPM |
Commodity |
Tolerances
originally requested by Syngenta
PPM |
|
sugarcane |
0.01 |
sugarcane |
0.01 |
| Cotton,
undelinted seed |
0.05 |
cottonseed |
0.05 |
| Cotton,
gin byproducts |
1.0 |
cotton
byproducts |
1.0 |
| Fruit,
citrus, Group 10
*see
below for fruits in this category |
0.03 |
citrus |
0.01 |
| almond
hulls |
0.01 |
- |
- |
| almond
|
0.02 |
almond
nut meat |
0.01 |
| tomatoe |
0.01 |
tomatoes |
0.01 |
| Fruit,
citrus, Group 10 includes - Ref: http://cfpub.epa.gov/oppref/food_feed/index.cfm |
| calamondin |
citrus,
oil |
lemon
|
orange,
sour, juice |
| citron,
citrus |
fruit
|
lemon,
dried pulp |
orange,
sour, oil |
| citrus |
fruit,
citrus |
lemon,
juice |
orange,
sweet |
| citrus
hybrids |
fruit,
citrus, dried pulp |
lemon,
oil |
orange,
sweet, dried pulp |
| citrus
hybrids, dried pulp |
fruit,
citrus, except mandarin |
lime |
orange,
sweet, juice |
| citrus
hybrids, juice |
fruit,
citrus, postharvest |
lime,
dried pulp |
orange,
sweet, oil |
| citrus
hybrids, oil |
grapefruit
|
lime,
juice |
pummelo |
| citrus,
dried pulp |
grapefruit,
dried pulp |
lime,
oil |
tangelo |
| citrus,
juice |
grapefruit,
juice |
mandarin,
satsuma |
tangerine |
| citrus,
meal |
grapefruit,
oil |
orange,
sour |
-- |
| citrus,
molasses |
kumquat |
orange,
sour, dried pulp |
| Some
excerpts from Table 1.-- Subchronic, Chronic, and Other
Toxicity |
| Sudy
Type and -[Guideline No.] |
Results |
| 90-Day
oral toxicity rodents (rats) - [870.3100] |
NOAEL: 507/549 milligrams/kilogram/day (mg/ kg/day) Male/Female
(M/F) LOAEL: 1052/1128 mg/kg/day (M/F):
M = decreased
body weight, decreased body weight gain, equivocal
increased testicular atrophy at end of recovery
phase;
F = decreased
body weight, decreased body weight gain, equivocal
slightly increased histopathology in liver
(single cell necrosis, focal necrosis, inflammation, hepatocellular
hypertrophy). |
|
90-Day oral toxicity rodents (mice)
- [870.3100] |
NOAEL:
1,023/1,507 mg/kg/day (M/F) LOAEL: >1,023/>1,507 mg/kg/day
(M/F):
M = not attained;
F = not attained. |
|
90-Day oral toxicity in nonrodents (dogs)
- [870.3150] |
NOAEL:
19.8/19.6 mg/kg/day (M/F) LOAEL: 164.2/167.3 mg/kg/day
(M/F):
M = decreased
body weight gain (20%), slight hematological effects,
clinical chemistry changes suggesting
hepatotoxicity, decreased thymus
weight, thymic atrophy, increased
glycogen in liver, hemorrhage
in mesenteric lymph nodes;
F = decreased
body weight gain (44%), anemia
with extramedullary hematopoiesis in
liver/ spleen and myeloidhyperplasia in bone
marrow, clinical chemistry changes suggesting hepatotoxicity,
decrease thymus weight,
thymic atrophy and hyaline tubular change in kidney. |
| 21/28-Day
dermal toxicity (rats) - [870.3200] |
NOAEL: 1,000/100 mg/kg/day (M/F) LOAEL: >1,000/1,000 mg/kg/day(M/F):
M = not attained;
F = decreased
body weight gain. No dermal irritation M/F. |
| Prenatal
developmental in rodents (rats)
- [870.3700] |
Maternal
NOAEL: 300 mg/kg/day Maternal LOAEL: 1,000 mg/kg/day based
on decreased food consumption during treatment, decreased
body weight gain during post-treatment. Developmental
NOAEL: 300 mg/kg/day Developmental LOAEL: 1,000 mg/kg/day
based on slight decrease in fetal
weight, increased skeletal
anomalies, increased poor/absent
skeletal ossification. |
| Prenatal
developmental in nonrodents (rabbit)
- [870.3700] |
Maternal
NOAEL: 100 mg/kg/day Maternal LOAEL: 250 mg/kg/day based
on increased mortality, increased
vaginal/ anal bleeding. Developmental NOAEL: 50 mg/kg/day
Developmental LOAEL: 100 mg/kg/day based on abnormally
shaped heart (one fetus at 100 mg/kg/day and 3
fetuses from 2 litters at 250 mg/kg/day).
-- In historical control data provided
by the registrant, there were no reported instances of
abnormally shaped hearts. |
| Reproduction
and fertility effects (rat) - [870.3800] |
Parental
systemic NOAEL: 78.8/83.5 mg/kg/ day
(M/F) Parental systemic LOAEL: 631/676 mg/kg/day (M/F)
based on decreased body weight
and gain as well as decreased food consumption.
Offspring systemic NOAEL: 78.8/83.5 mg/kg/ day (M/F) Offspring
systemic LOAEL: 631/676 mg/kg/day (M/F): decreased
pup weight and weight gain, decreased spleen
weight, thymus weight and
increased vaginal patency.
Reproductive NOAEL: 968/1,030 mg/kg/day (M/ F) Reproductive
LOAEL: >968/1,030 (M/F) |
| Chronic
toxicity rodents
(rat) - [870.4100]
Chronic
feeding/ carcinogenicity
rats - [870.4300]
Carcinogenicity
rats -
[870.4200] |
NOAEL: 82.6/23.7 mg/kg/day (M/F) LOAEL: 429/99.3 mg/kg/day
(M/F):
M = decreased
body weight and gains, decreased food consumption
and increased Leydig cell hyperplasia
in testes;
F = increased tubular atrophy
in kidneys. At 500 mg/kg/
day decreased body weight,
body weight gain, food consumption and increased tubular
atrophy in kidneys. Negative
for carcinogenicity in M and F. |
Carcinogenicity
mice - [870.4200] |
NOAEL:
854/112 mg/kg/day (M/F) LOAEL: >854/818 mg/kg/day (M/F):
M = not determined;
F = decreased
body weight,
body weight gain and food consumption.
Negative for carcinogenicity in M and F. |
Chronic
toxicity
dogs - [870.4100] |
NOAEL: 51.1/45.3 mg/kg/day (M/F) LOAEL: 123/121 mg/kg/day
(M/F):
M = gray- white foci in lungs,
fibrous thickening of lung
pleura, equivocal decreased body
weight gain;
F = equivocal increased incidence
and severity of chronic urinary
bladder inflammation. |
In
vitro unscheduled DNA synthesis (primary rat hepatocytes)
- [870.5500] |
Negative
response up to 250 [mu]g/mL. Cytotoxicity
at £=15.63 [mu]g/ mL. |
| Acute
neurotoxicity screening battery (rat)
- [870.6200] |
NOAEL:
<2,000 mg/kg/day (M/F) LOAEL: 2,000 mg/kg/day (M/F):
M and F = decreased motor activity on day 1, histopathological
lesions in nervous system tissues. |
|
Subchronic neurotoxicity screening battery (rat)
- [870.6200 ] |
NOAEL:
112/553 mg/kg/day (M/F) LOAEL: 472/1,128 mg/kg/day (M/F):
M = decreased
body weight, body weight gain and food consumption.;
F = decreased
body weight. |
--
Trifloxysulfuron will be registered
for use on the following non-dietary sites: Turf--golfcourses.
-- A developmental neurotoxicity study in rats is not required. |
| Aug
20, 2003 |
OPP-2003-0272 |
SYNGENTA:
Registration
Application for 3 pesticide products:
Products Containing Active Ingredients not Included in Any
Previously Registered Products
-- 1. File symbol: 100-RRGE. Applicant: Syngenta Crop Protection.
Product name: TSS
75 WG. Active ingredient: Trifloxysulfuron-sodium
at 75%. Proposed classification/Use: None. Control
of certain broadleaf weeds, sedges, and grasses in
almonds, citrus, cotton, sugarcane, and tomatoes.
-- 2. File symbol: 100-RRGG. Applicant: Syngenta Crop Protection.
Product name: Trifloxysulfuron-sodium
Technical; Active ingredient: Trifloxysulfuron-sodium
at 94%. Proposed classification/Use:
None. For formulation into herbicides; for use on almonds,
citrus, cotton, sugarcane, tomatoes, and turf.
-- 3. File symbol: 100-RRGU. Applicant: Syngenta Crop Protection.
Product name: Monument.
Active ingredient: Trifloxysulfuron-sodium
at 75%. Proposed classification/Use:
None. Control of certain broadleaf weeds,
sedges, and grasses in turf. |
| March
21, 2003 |
OPP-2003-0057 |
Pesticide
petition for residues in or on
the raw agricultural commodities sugarcane at 0.01 ppm, cottonseed
at 0.05 ppm, cotton by-products at 1.0 ppm, citrus at 0.01,
almond hulls at 0.01 ppm, almond nut meat at 0.01 ppm, and
tomatoes at 0.01 ppm.
-- In the rat teratology study, 300 and 1,000 mg/kg/day caused
maternal toxicity consisting of reduced
body weight and food consumption. Developmental toxicity
was secondary to maternal toxicity and consisted of slightly
reduced fetal body weights and an increase
in minor skeletal anomalies and variations.
The
NOAELs for maternal and developmental toxicity were both 30
mg/kg/day.
-- Subchronic toxicity... In a 3-month
rat feeding study the NOAEL was
65.7 mg/kg with hematologic
and liver effects noted.
In
a 3-month mouse
feeding study, the NOAEL was 67.9 mg/kg. Effects seen
were adaptive
liver effects.
In a 3-month feeding study in dogs
the NOAEL was 19.6 mg/kg and
hematopoietic and liver effects
were seen.
-- Chronic toxicity... body weight gain
was decreased by 16% in males at 122
mg/kg/ day... There was a tendency for
a decrease in the erythrocyte count, hemoglobin concentration
and hematocrit for both sexes at 122
mg/kg/day at the end of treatment, and for
males throughout the treatment period.
-- In a 2-year chronic toxicity and carcinogenicity [rat]
study... At terminal sacrifice,
the testes to body weight ratio was increased by 19%
in the 464 mg/kg/day group. Microscopical
examination revealed a non-dose responsive increase in the
incidence of kidney tubular atrophy
in the two top dose groups of female rats, and an increase
in Leydig cell hyperplasia in high dose males only...
The NOAEL in males was 82.6 mg/kg/day
based on the increased incidence of
Leydig cell hyperplasia, and 23.7 mg/kg/day
in females based on the increased
incidence of kidney tubular atrophy.
-- In a 90-day subchronic neurotoxicity
study in rats... based on body
weight effects, the NOAEL was established
at 112 mg/kg/day for male rats
and 553 mg/kg/day for female rats.
|
|