This section on the Thyroid includes:
• INTRODUCTION
Part 1: Fluoride Exposure Aggravates the Impact of Iodine Deficiency
Part 2: Fluoride and Goiter
Part 3: Fluoride’s Impact on Thyroid Hormones
Part 4: Fluoride Aggravates Thyroid Damage Caused by Excess Iodine Intake
Part 5: NRC (2006): Fluoride’s Impact on the Thyroid Gland
The following discussion is from pages 224-236 of the NRC’s report’s “Fluoride in Drinking Water: A Scientific Review of EPA’s Standards.”
Effects on the Endocrine System
The endocrine system, apart from reproductive aspects, was not considered in detail in recent major reviews of the health effects of fluoride (PHS 1991; NRC 1993; Locker 1999; McDonagh et al. 2000a; WHO 2002; ATSDR 2003). Both the Public Health Service (PHS 1991) and the World Health Organization (WHO 2002) mentioned secondary hyperparathyroidism in connection with discussions of skeletal fluorosis, but neither report examined endocrine effects any further. The Agency for Toxic Substances and Disease Registry (ATSDR 2003) discussed four papers on thyroid effects and two papers on parathyroid effects and concluded that “there are some data to suggest that fluoride does adversely affect some endocrine glands.” McDonagh et al. (2000a) reviewed a number of human studies of fluoride effects, including three that dealt with goiter and one that dealt with age at menarche. The following section reviews material on the effects of fluoride on the endocrine system—in particular, the thyroid (both follicular cells and parafollicular cells), parathyroid, and pineal glands. Each of these sections has its own discussion section. Detailed information about study designs, exposure conditions, and results is provided in Appendix E.
The follicular cells of the thyroid gland produce the classic thyroid hormones thyroxine (T4) and triiodothyronine (T3); these hormones modulate a variety of physiological processes, including but not limited to normal growth and development (Larsen et al. 2002; Larsen and Davies 2002; Goodman 2003). Between 4% and 5% of the U.S. population may be affected by deranged thyroid function (Goodman 2003), making it among the most prevalent of endocrine diseases (Larsen et al. 2002). The prevalence of subclinical thyroid dysfunction in various populations is 1.3-17.5% for subclinical hypothyroidism and 0.6-16% for subclinical hyperthyroidism; the reported rates depend on age, sex, iodine intake, sensitivity of measurements, and definition used (Biondi et al. 2002). Normal thyroid function requires sufficient intake of iodine (at least 100 micrograms/day [µg/d]), and areas of endemic iodine deficiency are associated with disorders such as endemic goiter and cretinism (Larsen et al. 2002; Larsen and Davies 2002; Goodman 2003). Iodine intake in the United States (where iodine is added to table salt) is decreasing (CDC 2002d; Larsen et al. 2002), and an estimated 12% of the population has low concentrations of urinary iodine (Larsen et al. 2002).
The principal regulator of thyroid function is the pituitary hormone thyroid-stimulating hormone (TSH), which in turn is controlled by positive input from the hypothalamic hormone thyrotropin-releasing hormone (TRH) and by negative input from T4 and T3. TSH binds to G-protein-coupled receptors in the surface membranes of thyroid follicular cells (Goodman 2003), which leads to increases in both the cyclic adenosine monophosphate (cAMP) and diacylglycerol/inositol trisphosphate second messenger pathways (Goodman 2003). T3, rather than T4, probably is responsible for the feedback response for TSH production (Schneider et al. 2001). Some T3, the active form of thyroid hormone, is secreted directly by the thyroid along with T4, but most T3 is produced from T4 by one of two deiodinases (Types I and II1) in the peripheral tissue (Schneider et al. 2001; Larsen et al. 2002; Goodman 2003). T3 enters the nucleus of the target cells and binds to specific receptors, which activate specific genes.
Background
An effect of fluoride exposure on the thyroid was first reported approximately 150 years ago (Maumené 1854, 1866; as cited in various reports). In 1923, the director of the Idaho Public Health Service, in a letter to the Surgeon General, reported enlarged thyroids in many children between the ages of 12 and 15 using city water in the village of Oakley, Idaho (Almond 1923); in addition, the children using city water had severe enamel deficiencies in their permanent teeth. The dental problems were eventually attributed to the presence in the city water of 6 mg/L fluoride, and children born after a change in water supply (to water with <0.5 mg/L fluoride) were not so affected (McKay 1933); however, there seems to have been no further report on thyroid conditions in the village.
More recently, Demole (1970) argued that a specific toxicity of fluoride for the thyroid gland does not exist, because (1) fluoride does not accumulate in the thyroid; (2) fluoride does not affect the uptake of iodine by thyroid tissue; (3) pathologic changes in the thyroid show no increased frequency in regions where water is fluoridated (naturally or artificially); (4) administration of fluoride does not interfere with the prophylactic action of iodine on endemic goiter; and (5) the beneficial effect of iodine in threshold dosage to experimental animals is not inhibited by administration of fluoride, even in excessive amounts. Bürgi et al. (1984) also stated that fluoride does not potentiate the consequences of iodine deficiency in populations with a borderline or low iodine intake and that published data fail to support the hypothesis that fluoride has adverse effects on the thyroid (at doses recommended for caries prevention). McLaren (1976), however, pointed out the complexity of the system, the difficulties in making adequate comparisons of the various studies of fluoride and the thyroid, and evidence for fluoride accumulation in the thyroid and morphological and functional changes (e.g., changes in activity of adenylyl cyclase), suggesting that analytical methods could have limited the definitiveness of the data to date. His review suggested that physiological or functional changes might occur at fluoride intakes of 5 mg/day.
Although fluoride does not accumulate significantly in most soft tissue (as compared to bones and teeth), several older studies found that fluoride concentrations in thyroid tissue generally exceed those in most other tissue except kidney (e.g., Chang et al. 1934; Hein et al. 1954, 1956); more recent information with improved analytic methods for fluoride was not located. Several studies have reported no effect of fluoride treatment on thyroid weight or morphology (Gedalia et al. 1960; Stolc and Podoba 1960; Saka et al. 1965; Bobek et al. 1976; Hara 1980), while others have reported such morphological changes as mild atrophy of the follicular epithelium (Ogilvie 1953), distended endoplasmic reticulum in follicular cells (Sundström 1971), and “morphological changes suggesting hormonal hypofunction” (Jonderko et al. 1983).
Fluoride was once thought to compete with iodide for transport into the thyroid, but several studies have demonstrated that this does not occur (Harris and Hayes 1955; Levi and Silberstein 1955; Anbar et al. 1959; Saka et al. 1965). The iodide transporter accepts other negatively charged ions besides iodide (e.g., perchlorate), but they are about the same size as iodide (Anbar et al. 1959); fluoride ion is considerably smaller and does not appear to displace iodide in the transporter.
Animal Studies
A number of studies have examined the effects of fluoride on thyroid function in experimental animals or livestock (for details, see Appendix E, Tables E-1, E-2, and E-3). Of these, the most informative are those that have considered both the fluoride and iodine intakes.
Guan et al. (1988) found that a fluoride intake of 10 mg/L in drinking water had little apparent effect on Wistar rats with sufficient iodine intake, but a fluoride intake of 30 mg/L in drinking water resulted in significant decreases in thyroid function (decreases in T4, T3, thyroid peroxidase, and 3H-leucine), as well as a decrease in thyroid weight and effects on thyroid morphology (Table E-2). In iodine-deficient rats, fluoride intake of 10 mg/L in drinking water produced abnormalities in thyroid function beyond that attributable to low iodine, including decreased thyroid peroxidase, and low T4 without compensatory transformation of T4 to T3.
Zhao et al. (1998), using male Kunmin mice, found that both iodine-deficient and iodine-excess conditions produced goiters, but, under iodine-deficient conditions, the goiter incidence at 100 days increased with increased intake of fluoride. At 100 days, the high-fluoride groups had elevated serum T4 at all concentrations of iodine intake and elevated T3 in iodine-deficient animals. High fluoride intake significantly inhibited the radioiodine uptake in the low- and normal-iodine groups.
Stolc and Podoba (1960) found a decrease in protein-bound iodine in blood in fluoride-treated female rats (3-4 mg/kg/day) fed a low-iodine diet but not in corresponding rats fed a larger amount of iodine. Both groups (low- and high-iodine) of fluoride-treated rats showed a reduced rate of biogenesis of T3 and T4 after administration of 131I compared with controls (Stolc and Podoba 1960).
Bobek et al. (1976) found decreases in plasma T4 and T3 as well as a decrease in free T4 index and an increase in T3-resin uptake in male rats given 0.1 or 1 mg of fluoride per day (0.4-0.6 or 4-6 mg/kg/day) in drinking water for 60 days.2 The authors suggested the possibility of decreased binding capabilities and altered thyroid hormone transport in blood.
Decreases in T4 and T3 concentrations have been reported in dairy cows at estimated fluoride doses up to 0.7 mg/kg/day with possible iodine deficiency (Hillman et al. 1979; Table E-3). Reduced T3 (Swarup et al. 1998) and reduced T3, T4, and protein-bound iodine (Cinar and Selcuk 2005) have also been reported in cows diagnosed with chronic fluorosis in India and Turkey, respectively.
Hara (1980) found elevated T3 and T4 at the lowest dose (approximately 0.1 mg/kg/day), decreased T3 and normal T4 at intermediate doses (3-4 mg/kg/day), and decreased TSH and growth hormone (indicating possible effects on pituitary function) at the highest doses (10-20 mg/kg/day). This was the only animal study of fluoride effects on thyroid function to measure TSH concentrations; however, full details (e.g., iodine intake) are not available in English.
Other studies have shown no effect of fluoride on the end points examined (Gedalia et al. 1960; Siebenhüner et al. 1984; Clay and Suttie 1987; Choubisa 1999; Table E-1). Choubisa (1999) looked only for clinical evidence of goiter in domestic animals (cattle and buffaloes) showing signs of enamel or skeletal fluorosis; no hormone parameters (e.g., T4, T3, TSH) were measured. Gedalia et al. (1960) also did not measure T4, T3, or TSH; radioiodine uptake, protein-bound iodine, and total blood iodine were all normal in rats receiving fluoride doses up to approximately 1 milligram per kilogram of body weight per day (mg/kg/day). Clay and Suttie (1987) reported no significant differences from control values for T4 concentration and T3 uptake in heifers fed up to 1.4 mg/kg/day; iodine intake is not stated but probably was adequate, and TSH was not measured.
Siebenhüner et al. (1984) carried out a special experiment involving iodine depletion of the thyroid before 6 days of fluoride treatment. No effects were seen on the parameters measured, including T3 and T4 concentrations; however, TSH was not measured. In addition, propylthiouracil (PTU), the agent used to deplete the thyroid of iodine, also has an inhibitory effect on deiodinases (Larsen et al. 2002; Larsen and Davies 2002); Siebenhüner et al. (1984) did not mention this second action of PTU and its relevance to the interpretation of the experimental results, and there was no control group without the PTU treatment.
Human Studies
Several authors have reported an association between endemic goiter and fluoride exposure or enamel fluorosis in human populations in India (Wilson 1941; Siddiqui 1960; Desai et al. 1993), Nepal (Day and Powell-Jackson 1972), England (Wilson 1941; Murray et al. 1948), South Africa (Steyn 1948; Steyn et al. 1955; Jooste et al. 1999), and Kenya (Obel 1982). Although endemic goiter is now generally attributed to iodine deficiency (Murray et al. 1948; Obel 1982; Larsen et al. 2002; Belchetz and Hammond 2003), some of the goitrogenic areas associated with fluoride exposure were not considered to be iodine deficient (Steyn 1948; Steyn et al. 1955; Obel 1982; Jooste et al. 1999). Obel (1982) indicated that many cases of fluorosis in Kenya occur concurrently with goiter. Several authors raise the possibility that the goitrous effect, if not due to fluoride, is due to some other substance in the water (e.g., calcium or water hardness) that was associated with the fluoride concentration (Murray et al. 1948; Day and Powell-Jackson 1972) or that enhanced the effect of fluoride (Steyn 1948; Steyn et al. 1955). Dietary selenium deficiencies (e.g., endemic in parts of China and Africa or due to protein-restricted diets) can also affect normal thyroid function3 (Larsen et al. 2002); no information on dietary selenium is available in any of the fluoride studies. Appendix E summarizes a number of studies of the effects of fluoride on thyroid function in humans (see Table E-4).
Three studies illustrated the range of results that have been reported: (1) Gedalia and Brand (1963) found an association between endemic goiter in Israeli girls and iodine concentrations in water but found no association with fluoride concentrations (<0.1-0.9 mg/L). (2) Siddiqui (1960) found goiters only in persons aged 14-17 years; the goiters, which became less visible or invisible after puberty, were associated with mean fluorine content of the water (5.4-10.7 mg/L) and were inversely associated with mean iodine content of the water. (3) Desai et al. (1993) found a positive correlation (P < 0.001) between prevalence of goiter (9.5-37.5%) and enamel fluorosis (6.0-59.0%), but no correlation between prevalence of goiter and water iodine concentration (P > 0.05).
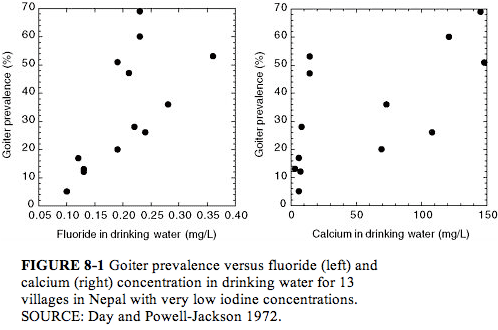
Day and Powell Jackson (1972) surveyed 13 villages in Nepal where the water supply was uniformly low in iodine (?1 µg/L; see Figure 8-1). Here the goiter prevalence (5-69%, all age groups) was directly associated with the fluoride concentration (<0.1 to 0.36 mg/L; P < 0.01) or with hardness, calcium concentration, or magnesium concentration of the water (all P < 0.01). Goiter prevalence of at least 20% was associated with all fluoride concentrations ? 0.19 mg/L, suggesting that fluoride might influence the prevalence of goiter in an area where goiter is endemic because of low iodine intake. The possibility of a nutritional component (undernutrition or protein deficiency) to the development of goiter was also suggested.
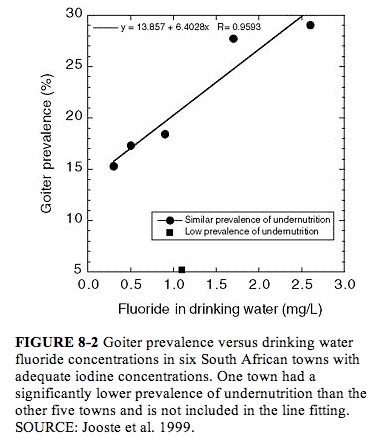
Jooste et al. (1999) examined children (ages 6, 12, and 15) who had spent their entire lives in one of six towns in South Africa where iodine concentrations in drinking water were considered adequate (median urinary iodine concentration exceeding 201 µg/L [1.58 µmol/L]; see Appendix E, Tables E-4 and E-5; Figure 8-2). For towns with low (0.3-0.5 mg/L) or near “optimal” (0.9-1.1 mg/L) fluoride concentrations in water, no relationship between fluoride and prevalence of mild goiter was found (5-18%); for the other two towns (1.7 and 2.6 mg/L fluoride), however, goiter prevalences were 28% and 29%, respectively, and most children had severe enamel mottling. These two towns (and one low-fluoride town) had very low proportions (0-2.2%) of children with iodine deficiency, defined as urinary iodine concentrations <100 µg/L (<0.79 µmol/L). The town with the lowest prevalence of goiter also had the lowest prevalence of undernutrition; the two towns with the highest prevalence of goiter (and highest fluoride concentrations) did not differ greatly from the remaining three towns with respect to prevalence of undernutrition. The authors suggested that fluoride or an associated goitrogen might be responsible for the goiters seen in the two towns with the highest fluoride concentrations but that some other factor(s) was involved in development of goiter in the other towns.
Several studies have compared various aspects of thyroid status in populations with different fluoride intakes (for details, see Appendix E, Table E-4). Leone et al. (1964) and Baum et al. (1981) reported no significant differences in thyroid status between populations with low (0.09-0.2 mg/L) and high (3-3.5 mg/L) fluoride concentrations in the drinking water. Leone et al. (1964) looked only at protein-bound iodine and physical examination of the thyroid in adults; Baum et al. (1981) measured a number of parameters in teenagers, including T4, T3, and TSH. Neither study reported iodine status of the groups. Baum et al. (1981) showed but did not explain a decrease in thyroglobulin in girls in the high-fluoride group.
Bachinskii et al. (1985) examined 47 healthy persons, 43 persons with hyperthyroidism, and 33 persons with hypothyroidism. Prolonged consumption of “high-fluoride” drinking water (2.3 mg/L, as opposed to “normal” concentrations of 1 mg/L) by healthy persons was associated with statistically significant changes in TSH concentrations (increased), T3 concentrations (decreased), and uptake of radioiodine (increased), although the mean values for TSH and T3 were still within normal ranges (see Appendix E, Table E-6). The mean value of TSH for the healthy group (4.3 ± 0.6 milliunits/L; Table E-6) is high enough that one expects a few individuals to have been above the normal range (typically 0.5-5 milliunits/L; Larsen et al. 2002). These results were interpreted as indicating disruption of iodine metabolism, stress in the pituitary-thyroid system, and increased risk of developing thyroidopathy (Bachinskii et al. 1985).
Lin et al. (1991) examined 769 children (7-14 years old) for mental retardation in three areas of China, including an area with “high” fluoride (0.88 mg/L) and low iodine, an area with “normal” fluoride (0.34 mg/L) and low iodine, and an area where iodine supplementation was routine (fluoride concentration not stated). Ten to twelve children in each area received detailed examinations, including measuring thyroid 131I uptake and thyroid hormone concentrations. Children in the first area had higher TSH, slightly higher 131I uptake, and lower mean IQ than children in the second area. Children in the first area also had reduced T3 and elevated reverse T3, compared with children in the second area. The authors suggested that high fluoride might exacerbate the effects of iodine deficiency. In addition, the authors reported a difference in T3/rT3 (T3/reverse-T3) ratios between high- and low-fluoride areas and suggested that excess fluoride ion affects normal deiodination.
A recent study by Susheela et al. (2005) compared thyroid hormone status (free T4, free T3, and TSH) of 90 children with enamel fluorosis (drinking water fluoride ranging from 1.1 to 14.3 mg/L) and 21 children without enamel fluorosis (0.14-0.81 mg/L fluoride in drinking water) in areas where iodine supplementation was considered adequate.4 Forty-nine children (54.4%) in the sample group had “well-defined hormonal derangements”; findings were borderline in the remaining 41 children. The types of hormonal derangements included elevated TSH and normal T4 and T3 (subclinical hypothyroidism); low T3 and normal T4 and TSH (“low T3 syndrome”); elevated T3 and TSH and normal T4 (possible T3 toxicosis); elevated TSH, low T4, and normal T3 (usually indicative of primary hypothyroidism and iodine deficiency); and low T3, high TSH, and normal T4. All but the first category are considered to be associated with or potentially caused by abnormal activity of deiodinases. The authors concluded that fluoride in excess may be inducing diseases that have usually been attributed to iodine deficiency and that iodine supplementation may not be adequate when excess fluoride is being consumed.
Thyroid hormone disturbances were also noted in the control children, and urine and fluoride concentrations in the control children reflect higher fluoride intake than can be accounted for by the drinking water alone (Susheela et al. 2005). Thus, the authors recommend that end points such as hormone concentrations should be examined with respect to serum or urinary fluoride concentrations, not just drinking water fluoride concentrations. In addition, they note that all hormone endpoints (T3, T4, and TSH) should be examined, lest some of the abnormalities be missed.
Mikhailets et al. (1996) detected thyroid abnormalities (moderate reduction of iodine uptake, low T3, normal T4, and increased TSH) in 165 aluminum workers with signs of chronic fluorosis and an estimated average fluoride intake of 10 mg/working day. A tendency toward increased TSH was observed with increased exposure time and with more severe fluorosis. Workers with more than 10 years of service had a significant decrease in T3 concentration in comparison to controls. The frequency of individuals with low concentrations of T3 (corresponding to hypothyroidism) was 65% among workers with more than 10 years of service and 54% among workers with Stage 2 fluorosis. The highest frequency of occurrence of low T3 (76%) was observed in people with chronic fluoride intoxication including liver damage (moderate cytolysis), suggesting a disorder in peripheral conversion of T4 to T3 (deiodination). The possibility of indirect effects of fluorine on enzymatic deiodination was also suggested.
Tokar? et al. (1989) and Balabolkin et al. (1995) have also reported thyroid effects in fluoride- or fluorine-exposed workers; full details of these studies are not available in English. Balabolkin et al. (1995) found that 51% of the workers examined had subclinical hypothyroidism with reduced T3.
No changes in thyroid function were detected in two studies of osteoporosis patients treated with NaF for 6 months or several years (Eichner et al. 1981; Hasling et al. 1987; for details, see Appendix E, Table E-7). These study populations are not necessarily representative of the general population, especially with respect to age and the fact that they usually receive calcium supplements. In an earlier clinical study to examine the reported effects of fluoride on individuals with hyperthyroidism, Galletti and Joyet (1958) found that, in 6 of 15 patients, both basal metabolic rate and protein-bound iodine fell to normal concentrations, and the symptoms of hyperthyroidism were relieved after fluoride treatment. Fluoride was considered clinically ineffective in the other 9 patients, although improvement in basal metabolic rate or protein-bound iodine was observed in some of them. In the 6 patients for whom fluoride was effective, tachycardia and tremor disappeared within 4-8 weeks, and weight loss was stopped. The greatest clinical improvement was observed in women between 40 and 60 years old with a moderate degree of thyrotoxicosis; young patients with the classic symptoms of Graves’ disease did not respond to fluoride therapy. Radioiodine uptake tests were performed on 10 of the patients, 7 of whom showed an inhibitory effect on initial 131I uptake by the thyroid.
Discussion (Effects on Thyroid Function)
In studies of animals with dietary iodine sufficiency, effects on thyroid function were seen at fluoride doses of 3-6 mg/kg/day (Stolc and Podoba 1960; Bobek et al. 1976; Guan et al. 1988; Zhao et al. 1998); in one study, effects were seen at doses as low as 0.4-0.6 mg/kg/day (Bobek et al. 1976). In low-iodine situations, more severe effects on thyroid function were seen at these doses (Stolc and Podoba 1960; Guan et al. 1988; Zhao et al. 1998). Effects on thyroid function in low-iodine situations have also been noted at fluoride doses as low as 0.06 mg/kg/day (Zhao et al. 1998), ?0.7 mg/kg/day (Hillman et al. 1979), and 1 mg/kg/day (Guan et al. 1988). Studies showing no effect of fluoride on thyroid function did not measure actual hormone concentrations (Gedalia et al. 1960; Choubisa 1999), did not report iodine intakes (Gedalia et al. 1960; Clay and Suttie 1987; Choubisa 1999), used fluoride doses (<1.5 mg/kg/day) below those (3-6 mg/kg/day) associated with effects in other studies (Gedalia et al. 1960; Clay and Suttie 1987), or did not discuss a possibly complicating factor of the experimental procedure used (Siebenhüner et al. 1984). Only one animal study (Hara 1980) measured TSH concentrations, although that is considered a “precise and specific barometer” of thyroid status in most situations (Larsen et al. 2002). Full details of Hara’s report are not available in English.
Goiter prevalence of at least 20% has been reported in humans exposed to water fluoride concentrations ? 0.2 mg/L (low-iodine situation; Day and Powell-Jackson 1972) or 1.5-3 mg/L (undernutrition, but adequate iodine; Jooste et al. 1999); however, other causes of goiter have not been ruled out. Bachinskii et al. (1985) showed increased TSH concentrations and reduced T3 concentrations in a population with a fluoride concentration of 2.3 mg/L in their drinking water (in comparison to a group with 1.0 mg/L), and Lin et al. (1991) showed similar results for a population with 0.88 mg/L fluoride in the drinking water (in comparison to a group with 0.34 mg/L); another study showed no effect at 3 mg/L (Baum et al. 1981). Among children considered to have adequate iodine supplementation, Susheela et al. (2005) found derangements of thyroid hormones in 54% of children with enamel fluorosis (1.1-14.3 mg/L fluoride in drinking water), and in 45-50% of “control” children without enamel fluorosis but with elevated serum fluoride concentrations. Mikhailets et al. (1996) observed an increase in TSH in workers with increased exposure time and with more severe fluorosis; low T3 was found in 65% of workers with more than 10 years of service and in 54% of workers with Stage 2 fluorosis. Several studies do not include measurements of T4, T3, or TSH (Siddiqui 1960; Gedalia and Brand 1963; Leone et al. 1964; Day and Powell-Jackson 1972; Teotia et al. 1978; Desai et al. 1993; Jooste et al. 1999).
Nutritional information (especially the adequacy of iodine and selenium intake) is lacking for many (iodine) or all (selenium) of the available studies on humans. As with the animal studies, high fluoride intake appears to exacerbate the effects of low iodine concentrations (Day and Powell-Jackson 1972; Lin et al. 1991). Uncertainty about total fluoride exposures based on water fluoride concentrations, variability in exposures within population groups, and variability in response among individuals generally have not been addressed. Although no thyroid effects were reported in studies using controlled doses of fluoride for osteoporosis therapy, the study populations are not necessarily representative of the general population with respect to age, calcium intake, and the presence of metabolic bone disease.
Thus, several lines of information indicate an effect of fluoride exposure on thyroid function. However, because of the complexity of interpretation of various parameters of thyroid function (Larsen et al. 2002), the possibility of peripheral effects on thyroid function instead of or in addition to direct effects on the thyroid, the absence of TSH measurements in most of the animal studies, the difficulties of exposure estimation in human studies, and the lack of information in most studies on nutritional factors (iodine, selenium) that are known to affect thyroid function, it is difficult to predict exactly what effects on thyroid function are likely at what concentration of fluoride exposure and under what circumstances.
Suggested mechanisms of action for the results reported to date include decreased production of thyroid hormone, effects on thyroid hormone transport in blood, and effects on peripheral conversion of T4 to T3 or on normal deiodination processes, but details remain uncertain. Both peripheral conversion of T4 to T3 and normal deiodination (deactivation) processes require the deiodinases (Types I and II for converting T4 to T3 and Types I and III for deactivation; Schneider et al. 2001; Larsen et al. 2002; Goodman 2003). Several sets of reported results are consistent with an inhibiting effect of fluoride on deiodinase activity; these effects include decreased plasma T3 with normal or elevated T4 and TSH and normal T3 with elevated T4 (Bachinskii et al. 1985; Guan et al. 1988; Lin et al. 1991; Balabolkin et al. 1995; Michael et al. 1996; Mikhailets et al. 1996; Susheela et al. 2005). The antihyperthyroid effect that Galletti and Joyet (1958) observed in some patients is also consistent with an inhibition of deiodinase activity in those individuals.
The available studies have generally dealt with mean values of various parameters for the study groups, rather than with indications of the clinical significance, such as the fraction of individuals with a value (e.g., TSH concentration) outside the normal range or with clinical thyroid disease. For example, in the two populations of asymptomatic individuals compared by Bachinskii et al. (1985), the elevated mean TSH value in the higher-fluoride group is still within the normal range, but the number of individuals in that group with TSH values above the normal range is not given.
In the absence of specific information in the reports, it cannot be assumed that all individuals with elevated TSH or altered thyroid hormone concentrations were asymptomatic, although many might have been. For asymptomatic individuals, the significance of elevated TSH or altered thyroid hormone concentrations is not clear. Belchetz and Hammond (2003) point out that the population-derived reference standards (e.g., for T4 and TSH) reflect the mean plus or minus two standard deviations, meaning that 5% of normal people have results outside a given range. At the same time, healthy individuals might regulate plasma T4 within a “personal band” that could be much more narrow than the reference range; this brings up the question of whether a disorder shifting hormone values outside the personal band but within the population reference range requires treatment (Davies and Larsen 2002; Belchetz and Hammond 2003). For example, early hypothyroidism can present with symptoms and raised TSH but with T4 concentrations still within the reference range (Larsen et al. 2002; Belchetz and Hammond 2003).
Subclinical hypothyroidism is considered a strong risk factor for later development of overt hypothyroidism (Weetman 1997; Helfand 2004). Biondi et al. (2002) associate subclinical thyroid dysfunction (either hypo or hyperthyroidism) with changes in cardiac function and corresponding increased risks of heart disease. Subclinical hyperthyroidism can cause bone demineralization, especially in postmenopausal women, while subclinical hypothyroidism is associated with increased cholesterol concentrations, increased incidence of depression, diminished response to standard psychiatric treatment, cognitive dysfunction, and, in pregnant women, decreased IQ of their offspring (Gold et al. 1981; Brucker-Davis et al. 2001). Klein et al. (2001) report an inverse correlation between severity of maternal hypothyroidism (subclinical or asymptomatic) and the IQ of the offspring (see also Chapter 7).
A number of authors have reported delayed eruption of teeth, enamel defects, or both, in cases of congenital or juvenile hypothyroidism (Hinrichs 1966; Silverman 1971; Biggerstaff and Rose 1979; Noren and Alm 1983; Loevy et al. 1987; Bhat and Nelson 1989; Mg’ang’a and Chindia 1990; Pirinen 1995; Larsen and Davies 2002; Hirayama et al. 2003; Ionescu et al. 2004). No information was located on enamel defects or effects on eruption of teeth in children with either mild or subclinical hypothyroidism. The possibility that either dental fluorosis (Chapter 4) or the delayed tooth eruption noted with high fluoride intake (Chapter 4; see also Short 1944) may be attributable at least in part to an effect of fluoride on thyroid function has not been studied.
. . . .
Recommendations
. . . .
The effects of fluoride on various aspects of endocrine function should be examined further, particularly with respect to a possible role in the development of several diseases or mental states in the United States. Major areas for investigation include the following:
- thyroid disease (especially in light of decreasing iodine intake by the U.S. population).