Abstract
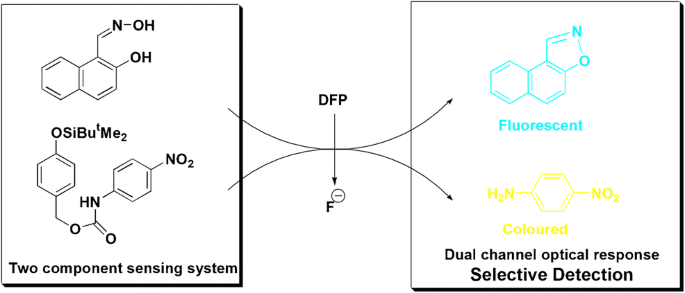
This paper reports a novel optical chemical sensing system for selective detection of diisopropylfluorophosphate (DFP), a simulant of fluorine-containing nerve agents (Sarin and Soman). Contrary to the reported methods involving only single sensing probe, this sensing system is comprised of two molecular sensing probes (1 and 2) having intrinsic affinities for reactive subunits of DFP (electrophilic phosphorus and fluoride ion). On exposure to DFP, two molecular probes react in tandem with electrophilic phosphorus and fluoride ion (by-product of the initial phosphorylation reaction) to induce a unique modulation in the optical properties of the sensing system which leads to selective detection of DFP in solution as interferents like phosphorus-containing compounds, acids, and anions were unable to induce similar optical modulation due to lack of both electrophilic phosphorus and fluorine in the same molecule. Calibration curve between the amount of DFP added and the absorption intensity revealed the colorimetric detection limit of the system to be 4.50 ?M which was further lowered to 2.22 ?M by making use of a self-immolative fluoride sensing probe 5.
Graphical abstract
References
-
Somani SM. Chemical warfare agents. San Diego: Academic Press; 1992.
-
Investigative Science and Technology, Report of the scientific advisory body of temporary advisory group. Organisation for the Prohibition of Chemical Weapons. 2019, pp. 16–78.
-
Huixiang W, Danqun H, Yanan Z, Na M, Jingzhou H, Miao L, et al. A non-enzymatic electro-chemical sensor for organophosphorus nerve agent mimics and pesticides detection. Sensors Actuators B Chem. 2017;252:1118–24.
-
Pohanka M, Adam V, Kizek R. An acetylcholinesterase based chronoamperometric biosensor for fast and reliable assay of nerve agents. Sensors. 2013;13:11498–506.
-
Porzio M, Bettazzi F, Mandrich L, Giudice ID, Restaino OF, Laschi S, et al. Innovative biocatalysts as tools to detect and inactivate nerve agents. Nat Sci Rep. 2018;8:13773–84.
-
Buchapudi KR, Huang X, Yang X, Ji HF, Thundat T. Microcantilever biosensors for chemicals and bioorganisms. Analyst. 2011;136:1539–56.
-
Makinen MA, Anttalainen OA, Sillanpaa MET. Ion mobility spectrometry and its applications in detection of chemical warfare agents. Anal Chem. 2010;82:9594–600.
-
Puton J, Namiesnik J. Ion mobility spectrometry: current status and application for chemical warfare agents detection. Trends Anal Chem. 2016;85:10–20.
-
Kittle JD, Fisher BP, Esparza AJ, Morey AM, Iacono ST. Sensing chemical warfare agent simulants via photonic crystals of the Morpho didius butterfly. ACS Omega. 2017;2:8301–7.
-
Panneerselvam G, Thirumal V, Pandya HM. Review of surface acoustic wave sensors for the detection and identification of toxic environmental gases/vapours. Arch Acoust. 2018;43:357–67.
-
Diauudin FN, Rashid JIA, Knight VF, Yunus WMZW, Ong KK, Kasim NAM, et al. A review of current advances in the detection of organophosphorus chemical warfare agents based biosensor approaches. Sens Biosensing Res. 2019;26:100305–13.
-
Cai Y-C, Li, Song Q-H. Fluorescent chemosensors with varying degrees of intramolecular charge transfer for detection of a nerve agent mimic in solutions and in vapor. ACS Sens. 2017;2:834–41.
-
Pitschmann V, Matejovský L, Lunerová K, Dymák M, Urban M, Králík L. Detection papers with chromogenic chemosensors for direct visual detection and distinction of liquid chemical warfare agents. Chemosensors. 2019;7:30–41.
-
Ali SS, Gangopadhyay A, Maiti K, Mondal S, Pramanik AK, Guria UN, et al. A chromogenic and ratiometric fluorogenic probe for rapis detection of a nerve agent simulant DCP based on a hybrid hydoxynaphthalene-hemicyanine dye. Org Biomol Chem. 2017;15:5959–67.
-
Barba-Bon A, Costero AM, Gil S, Harriman A, Sancenon F. Highly selective detection of nerve-agent simulants with BODIPY dyes. Chem Eur J. 2014;20:6339–47.
-
Bui MPN, Abbas A. Simple and rapid colorimetric detection of p-nitrophenyl substituent organophosphorous nerve agents. Sensors Actuators B Chem. 2015;207:370–4.
-
Xu H, Zhang H, Zhao L, Peng C, Liu G, Cheng T. Naphthalimide-based fluorescent probe for highly sensitive and selective detection of nerve agent mimic DCP in solution and vapor phase. New J Chem. 2020;44:10713–8.
-
Lei Z, Yang Y. A concise colorimetric and fluorimetric probe for Sarin related threats designed via the “covalent-assembly” approach. J Am Chem Soc. 2014;136:6594–7.
-
Chen L, Wu D, Yoon J. Recent advances in the development of chromophore based chemosensors for nerve agents and phosgene. ACS Sens. 2018;3:27–43.
-
Belger C, Weis JG, Egap E, Swager TM. Colorimetric stimuli-responsive hydrogel polymers for the detection of nerve agent surrogates. Macromolecules. 2015;48:7990–4.
-
Kangas MJ, Ernest A, Lukowicz R, Mora AV, Quossi A, Perez M, et al. The identification of seven chemical warfare mimics using a colorimetric array. Sensors. 2018;18:4291–8.
-
Ohrui Y, Nagoya T, Kurimata N, Sodeyama M, Seto Y. Identification of V-type nerve agents in vapor samples using a field-portable capillary gas chromatography/membrane-interfaced electron ionization quadrupole mass spectrometry instrument with Tri-Bed concentrator and fluoridating conversion tube. J Mass Spectrom. 2017;52:472–9.
-
Zhou X, Lee S, Xu Z, Yoon J. Recent progress on the development of chemosensors for gases. Chem Rev. 2015;115:7944–8000.
-
Callan JF, de Silva AP, Magri DC. Luminescent sensors and switches in the early 21st century. Tetrahedron. 2005;61:8551–88.
-
Moragues ME, M-Manez R, Sancenon F. Chromogenic and fluorogenic chemosensors and reagents for anions. A comprehensive review of the year 2009. Chem Soc Rev. 2011;40:2593–643.
-
Lau YH, Rutledge PJ, Watkinson M, Todd MH. Chemical sensors that incorporate click-derived triazoles. Chem Soc Rev. 2011;40:2848–66.
-
L-Chruscinska E. Tetrazole peptides as copper (II) ion chelators. Coord Chem Rev. 2011;255:1824–33.
-
Jang YJ, Kim K, Tsay OG, Atwood DA, Churchill DG. Update 1 of destruction and detection of chemical warfare agents. Chem Rev. 2015;115:PR1–PR76.
-
Sambrook MR, Notman S. Supramolecular chemistry and chemical warfare agents: from fundamentals of recognition to catalysis and sensing. Chem Soc Rev. 2013;42:9251–67.
-
Tsuyoshi M. Design of Supramolecular sensors and their applications to organic chips and designs. Bull Chem Soc Jpn. 2021;94:24–33.
-
Hewage HS, Wallace KJ, Anslyn EV. Novel chemiluminescent detection of chemical warfare stimulant. Chem Commun. 2007:3909–11.
-
Barba-Bon A, Costero AM, Gil S, Martínez-Máñez R, Sancenón F. Selective chromo-fluorogenic detection of DFP (a Sarin and Soman mimic) and DCNP (a Tabun mimic) with a unique probe based on a boron dipyrromethene (BODIPY) dye. Org Biomol Chem. 2014;12:8745–51.
-
Gotor R, Costero AM, Gavina P, Gil S. Ratiometric double channel borondipyrromethene based chemodosimeter for the selective detection of nerve agent mimics. Dyes Pigments. 2014;108:76–83.
-
Royo S, Gotor R, Costero AM, Parra M, Gil S, Martínez-Máñez R, et al. Arylcarbinols as nerve agent probes. Influence of the conjugation in the sensing properties. New J Chem. 2012;36:1485–9.
-
Gotor R, Gavina P, Ochando LE, Chulvi K, Lorente A, Martinez-Manez R, et al. BODIPY dyes functionalized with 2-(2-dimethylaminophenyl)ethanol moieties as selective OFF–ON fluorescent chemodosimeters for the nerve agent mimics DCNP and DFP. RSC Adv. 2014;4:15975–82.
-
Sayed SE, Pascual L, Agostini A, Martnez-Manez R, Sancenon F, Costero AM, et al. A chromogenic probe for the selective recognition of Sarin and Soman mimic DFP. ChemistryOpen. 2014;3:142–5.
-
Sun X, Dahlhauser SD, Anslyn EV. A new auto-inductive cascade for the optical sensing of fluoride: application in the detection of phosphoryl fluoride nerve agents. J Am Chem Soc. 2017;139:4635–8.
-
Dale TJ, Rebek J. Hydroxy oximes as organophosphorus nerve agent sensors. Angew Chem Int Ed. 2009;48:7850–2.
-
Feigenbaum RP, Sella E, Shabat D. Autoinductive exponential signal amplification: a diagnostic probe for direct detection of fluoride. Chem Eur J. 2011;17:12123–8.
-
Dale TJ, Sather AC, Rebek J. Synthesis of novel aryl-1,2-oxazoles from ortho-hydroxyaryloximes. Tetrahedron Lett. 2009;50:6173–5.
-
Roth ME, Green O, Gnaim S, Shabat D. Dendritic, oligomeric, and polymeric self-immolative molecular amplification. Chem Rev. 2016;116:1309–52.
-
Amir RJ, Danieli E, Shabat D. A novel opposition-based tuned-chaotic differential evolution technique for techno economic analysis by optimal placement of distributed generation. Chem Eur J. 2007;13:812–21.
-
Zhu L, Anslyn EV. Signal amplification by allosteric catalysis. Angew Chem Int Ed. 2006;45:1190–6.
-
Investigative Science and Technology, Report of the scientific advisory body of temporary advisory group. Organisation for the Prohibition of Chemical Weapons. 2019, pp. 43.
Acknowledgements
The authors sincerely thank Director, DRDE, Gwalior, for his keen assistance and support (DRDE Accession No: DRDE/SC/30/2020).
