Abstract
The purpose was to discover whether fluoride (F) accumulates in the aged human pineal gland. The aims were to determine (a) F-concentrations of the pineal gland (wet), corresponding muscle (wet) and bone (ash); (b) calcium-concentration of the pineal. Pineal, muscle and bone were dissected from 11 aged cadavers and assayed for F using the HMDS-facilitated diffusion, F-ion specific electrode method. Pineal calcium was determined using atomic absorption spectroscopy. Pineal and muscle contained 297±257 and 0.5±0.4 mg F/kg wet weight, respectively; bone contained 2,037±1,095 mg F/kg ash weight. The pineal contained 16,000±11,070 mg Ca/kg wet weight. There was a positive correlation between pineal F and pineal Ca (r = 0.73, p<0.02) but no correlation between pineal F and bone F. By old age, the pineal gland has readily accumulated F and its F/Ca ratio is higher than bone.
Key Words: Calcium, Distribution, Fluoride, Human pineal gland, Hydroxyapatite, Pineal concretions
The pineal gland is a small organ situated near the centre of the brain. It is intimately related to the third ventricle. It is composed of pinealocytes and neuroglial cells amongst which ramifies a rich network of capillaries and postganglionic nerve fibres. The pineal gland is a mineralizing tissue. Its calcified concretions range from a few micrometres to several millimetres in diameter. The larger ones are identifiable on skull X-rays, cranial CT and MRI scans. The concretions are composed of hydroxyapatite (HA) [Angervall et al., 1958; Earle, 1965; Mabie and Wallace, 1974; Galliani et al., 1990; Bocchi and Valdre, 1993] whose chemical composition, morphology, and unit cell dimensions are similar to HA in bone and teeth [Mabie and Wallace, 1974; Bocchi and Valdre, 1993]. The pineal (calcified and uncalcified) has a high trace element content (zinc, iron, manganese, magnesium, strontium and copper) in humans [Krstic, 1976; Michotte et al., 1977] and in rats [Humbert and Pévet, 1991, 1996]. Michotte et al. [1977] suggested that, within the pineal, there are areas which are heavily loaded with calcium and which attract trace elements, even though these calcium-rich areas are not yet identifiable as concretions. Calcium is distributed throughout the pinealocytes: in the mitochondria, Golgi apparatus, cytoplasm, and nucleus [Krstic, 1976, 1995; Welsh, 1984; Pizarro et al., 1989, Lewczuk et al., 1994].
Fluoride does not accumulate in brain. Of all tissues, brain has the lowest fluoride concentration [Jenkins, 1991; Whitford, 1996; Ekstrand, 1996]. It is generally agreed that the blood-brain barrier restricts the passage of fluoride into the central nervous system. The human pineal gland is outside the blood-brain barrier [Arendt, 1995]. It is one of a few unique regions in the brain (all midline structures bordering the third and fourth ventricles) where the blood-brain barrier is weak. Cells in these regions require direct and unimpeded contact with blood [Rapoport, 1976]. Therefore, pinealocytes have free access to fluoride in the bloodstream. This fact, coupled with the presence of HA, suggest that the pineal gland may sequester fluoride from the bloodstream.
The purpose of this study was to discover whether fluoride accumulates in the aged pineal gland. Its objectives were to determine (a) the fluoride concentrations of the pineal gland (wet), corresponding muscle (wet) and bone (ash); (b) the pineal concentrations of calcium and HA.
Materials and Methods
The pineal glands and corresponding bone and muscle samples were dissected from I I aged cadavers (7 females and 4 males) in the Anatomy Department, UCL. The mean age was 82 years (range 70-100).
Preparation of the Samples
The pineal glands were blotted dry with tissue paper, weighed to the nearest milligram, homogenized in I ml double-distilled water using an agate pestle and mortar and sonicated for 10 min. Each pineal was divided into two portions that were analysed separately. Muscle samples weighing about 100 mg were treated likewise. Bone samples were cleaned of any adherent soft tissue with a razor blade, dried overnight at 110’C in an oven, and ashed (in porcelain crucibles with no fixatives) at 550-600°C in a muffle furnace for 8 h. Bone ash was pulverized into a fine powder using an agate pestle and mortar. Bone solutions were made by dissolving known weights of bone ash in 3 ml 2 M HC104. Bone solutions were analysed for fluoride in replicates of six and the mean fluoride concentrations in bone were calculated.
Determination of the Fluoride Concentrations
The homogenized pineal, homogenized muscle and bone solutions were assayed for fluoride using the HMDS-facilitated diffusion, F-ion-specific electrode method originally described by Taves [19681 and modified by Whitford and Reynolds [1979]. The protocol was further modified for use with the pineal glands. The concentration and volume of the base trap were increased to 0.5 M NaOH and 100 µl, respectively; the strength of the acetate buffer was increased to 50 µl 2 M acetic acid and the volume of the analysed solution was adjusted to 150 µl with double-distilled water. Diffusion time for pineal and muscle was 3 days; for bone 18 h.
Standards: pineal: 1,000, 2,500 and 5,000 nmol F; muscle: 5, 50 and 500 nmol F; bone: 10, 50, 100 and 500 nmol F.
Determination of the Concentration of Total Calcium in the Human Pineal Gland
The acid digests, which remained in the Petri dishes following the separation of fluoride from the pineal glands, were wet-acid ashed to decompose the organic component. The acid digests were placed in clean glass tubes and I ml conc. HN03 was added. The tubes were heated slowly to 50°C and maintained at 50°C for 30 min in a fume cupboard. The procedure was repeated using I ml 60% HC104. Two millilitre of double-distilled water was added to each tube and the volumes were measured. Calcium concentration was determined using atomic absorption spectroscopy.
Statistical Methods
Results were expressed as means ± SD. Differences between the groups were tested for significance using unpaired Student’s t-test. Differences were regarded as statistically significant when p<0.05.
Pearson’s correlation coefficient was used to test association between pineal fluoride and pineal calcium; and pineal fluoride and bone fluoride.
Results
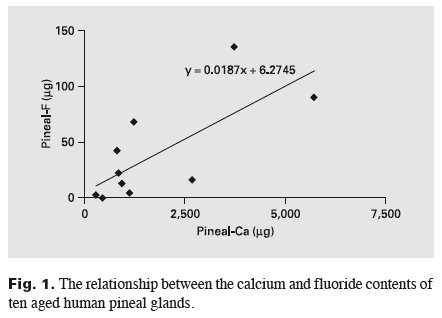
The aged pineal gland weighed 112±52 mg (56-198 mg). Pineal had a significantly higher F concentration than muscle: 297 ± 257 (14-875) vs. 0.5 ± 0.4 (0.2-1.5) mg F/kg wet weight (p<0.001). Bone contained 2,037± 1,095 (838-3,711) mg F/kg ash weight. The mean coefficient of variation between the replicates of F contents of bone solutions was 2.5 ± 1. 1 %. There was no correlation between pineal F and bone F. The pineal gland contained 16,000±11,070 (4,600-37,250) mg Ca/kg wet weight. Pineal fluoride and pineal calcium were directly correlated: r = 0.73, p < 0.02, n = 10, slope= 0.02 (fig. 1). Assuming stoichiometric HA, the pineal contained an estimated 40,000 ± 27,700 mg HA/kg wet weight (11,600-93,200 mg HA/kg). The estimated F concentration of pineal HA was 9,000 ± 7,800 mg/kg (650-21,800 mg/kg). Figure 2 shows that the F/calcium ratio was higher in pineal HA than in corresponding bone HA.
Discussion
This study has added new knowledge on the fate and distribution of fluoride in the body. It has shown for the first time that fluoride readily accumulates in the human pineal gland although there was considerable inter-individual variation (14-875 mg F/kg). By old age, the average pineal gland contains about the same amount of fluoride as teeth (300 mg F/kg) since dentine and whole enamel contain 300 and 100 mg F/kg, respectively [Newbrun, 1986]. Unlike brain capillaries, pineal capillaries allow the free passage of fluoride through the endothelium. If there had been a bloodbrain barrier in the pineal, it would have prevented the passage of fluoride into the pinealocytes and the pineal fluoride content would have been similar to or lower than muscle. This was obviously not the case: the fluoride concentration of the pineal was significantly higher (p<0.001) than muscle. The high fluoride levels in the pineal are presumably due to the large surface area of the HA crystallites both intra- and extracellularly. In addition, the pineal has a profuse blood flow and high capillary density; pineal blood flow (4 ml/min/g) is second only to the kidney [Arendt, 19951.
The extent of pineal calcification also varied between individuals: ranging from 4,600 to 37,250 mg Ca/kg wet weight. One of the aged pineals had very little precipitation. This supports the age independence of pineal calcification and agrees with previous studies [Cooper, 1932; Arieti, 1954; Tapp and Huxley, 197 1; Hasegawa et aL, 1987; Galliani et al., 1990]. The estimated fluoride concentration of pineal HA was 9,000 ± 7,800 mg/kg. The F/Ca ratio was higher in pineal HA than in corresponding bone (fig. 2). The extremely high level of substitution in the crystal structure of pineal HA by fluoride illustrates the readiness with which fluoride replaces the hydroxyl ion in the HA crystal. By old age, pineal HA has a higher fluoride content than other biological apatites. Unlike pineal concentrations of magnesium, manganese, zinc and copper, which, although very high, were generally within the limits found in bone and teeth [Michotte et al., 1977].
There was no correlation between pineal fluoride and bone fluoride. Therefore, unlike bone, pineal fluoride concentrations are not indicators of long-term fluoride exposure and body burden. Pineal fluoride, however, was significantly correlated with pineal calcium.
The methodology used in this project was accurate because the F values obtained for bone and muscle agreed with literature values. For example, the mean fluoride concentration of bone from elderly subjects was 2,000 mg/kg ash weight which agrees with previous studies using bone from subjects of a similar age [Ebie et al., 1992; Charen et al., 1979; Zipkin et al., 1958]. In this study, muscle contained 0.5 mg F/kg wet weight, a typical fluoride concentration for soft tissue [WHO, 1984]. The pineal and bone were treated differently during sample preparation (the pineal was wet-acid ashed, bone was dry ashed) which may somewhat obscure a direct comparison of the fluoride contents of pineal HA and bone. However, it is unlikely that there would be a significant analytical error.
In conclusion, this study presented evidence that fluoride readily accumulates in the aged pineal. Fluoride may also accumulate in a child’s pineal because significant amounts of calcification have been demonstrated in the pineals from young children [Cooper, 1932; Wurtman, 1968; Kerényi and Sarkar, 1968; Tapp and Huxley, 197 1; Doskocil, 1984]. In fact, calcification of the developing enamel organs and the pineal gland occur concurrently. If fluoride does accumulate in the child’s pineal (this needs verification), the pinealocytes will be exposed to relatively high local concentrations of fluoride. This could affect pineal metabolism in much the same way that high local concentrations of fluoride in the developing enamel organ affect ameloblast function. Research is presently underway to discover whether fluoride affects pineal physiology during childhood: specifically pineal synthesis of melatonin.
Acknowledgements
I acknowledge the Anatomy Deptartment, UCL, for providing the pineal glands; Prof. Gary Whitford, Medical College of Georgia, USA, and Gordon Hartman, University of Surrey, for their assistance with the fluoride analysis; Nicholas Porter, The Royal Surrey County Hospital, Guildford, for help with the determination of pineal-calcium concentrations. The Colt Foundation, The Heinz and Anna Kroch Foundation and The New Moorgate Trust funded the research.
References
- Angervall L, Berger S, Röckert H: A microradiographic and X-ray crystallographic study of calcium in the pineal body and intracranial tumours. Acta Pathol Microbiol Scand 1958;44: 113-119.
- Arendt J: Melatonin and the Mammalian Pineal Gland, ed 1. London. Chapman & Hall, 1995, p 17.
- Arieti S: The pineal gland in old age. J Neuropathol Exp Neurol 1954; 13:482-49 1.
- Bocchi G, Valdre G: Physical, chemical, and mineralogical characterization of carbonate-hydroxyapatite concretions of the human pineal gland. J Inorg Biochem 1993;49:209-220.
- Charen J, Taves DR, Stamm JW, Parkins FM: Bone fluoride concentrations associated with fluoridated drinking water. Calcif Tiss Int 1979;27: 95-99.
- Cooper ERA: The human pineal gland and pineal cysts. J Anat (Lond) 1932;67:28-46.
- Doskocil M: Development of concrements in the human pineal body. Folia Morphol (Praha) 1984;32:16-26.
- Earle KM: X-ray diffraction and other studies of the calcareous deposits in human pineal glands. J Neuropathol Exp Neurol 1965;4: 108-118.
- Ebie DM, Deaton TG, Wilson FC, Bawden JW: Fluoride concentrations in human and rat bone. J Public Health Dent 1992;52:288-29).
- Ekstrand J: Fluoride metabolism; in Fejerskov 0, Ekstrand J, Burt B (eds): Fluorides in Dentistry. Munksgaard, Copenhagen, 1996, pp 55-68.
- Galliani 1, Falcieri E, Giangaspero F, Valdre G, Mongiorgi R: A preliminary study of human pineal gland concretions: Structural and chemical analysis. Boll Soc Ital Biol Sper 1990;66: 615-622.
- Hasegawa A, Ohtsubo K, Mori W: Pineal gland in old age; quantitative and qualitative morphological study of 168 human autopsy cases. Brain Res 1987;409:343-349.
- Humbert W, Pévet P: Calcium content and concretions of pineal glands of young and old rats, Cell Tissue Res 199 1;263:593-596.
- Humbert W, Pévet P: Electron probe x-ray microanalysis of the elemental composition of the pineal gland of young adults and aged rats. J Pineal Res 1996;20:39-44.
- Jenkins GN: Physiology of fluoride; in Murray JJ, Rugg-Gunn AJ, Jenkins GN (eds): Fluorides in Caries Prevention, ed 3. London, Butterworth-Heinemann, 199 1, pp 262-294.
- Kerényi NA, Sarkar K: The postnatal transformation of the pineal gland. Acta Morphol Acad Sci Hung 1968; 16:223-236.
- Krstic R: A combined scanning and transmission electron microscopic study and electron probe microanalysis of human pineal acervuli. Cell Tiss Res 1976; 174:129-137.
- Krstic R: Ultracytochemical localization of calcium in the superficial pineal gland of the Mongolian gerbil. J Pineal Res 1985;2:21-37.
- Lewczuk B, Przybylska B, Wyrzykowski Z: Distribution of calcified concretions and calcium ions in the pig pineal gland. Folia Histochem Cytobiol 1994;32:243-249.
- Mabie CP, Wallace BM: Optical, physical and chemical properties of pineal gland calcifications. Calcif Tissue Res 1974; 16:59-7 1.
- Michotte Y, Lowenthal A, Knaepen L, Collard M, Massart DL: A morphological and chemical study of calcification of the pineal gland. J Neurol 1977;215:209-219.
- Newbrun E: Fluorides and Dental Caries, ed 3. Springfield, Thomas, 1986.
- Pizarro MDL, Gil JAL, Vasallo JL, Munoz Barragan L: Distribution of calcium in the pineal glands of normal rats. Adv Pineal Res 1989; 3:39-42.
- Rapoport SI: Blood-Brain Barrier in Physiology and Medicine. New York, Raven Press, 1976, p 77-78.
- Tapp E, Huxley M: The weight and degree of calcification of the pineal gland. J Pathol 197 1; 105:31-39.
- Taves DR: Separation of F by rapid diffusion using hexamethyldisiloxane. Talanta 1968;15:969-974.
- Welsh MG: Cytochemical analysis of calcium distribution in the superficial pineal gland of the Mongolian gerbil. J Pineal Res 1984;1:305-316.
- Whitford GM: The Metabolism and Toxicity of Fluoride. Monogr Oral Sci, 16, ed 2. Basel, Karger, 1996.
- Whitford GM, Reynolds KE: Plasma and developing enamel fluoride concentrations during chronic acid-base disturbances. J Dent Res 1979;58:2058-2065.
- WHO: Fluorine and Fluorides. Environmental Health Criteria 36. Geneva, WHO, 1984.
- Wurtman RJ: The pineal gland; in Endocrine Pathology. Baltimore, Williams & Wilkins, 1968, pp 117-132.
- Zipkin 1, McClure FJ, Leone NC, Lee WA: Fluoride deposition in human bones after prolonged ingestion of fluoride in drinking water. US Public Health Rep 1958;73:732-740.

