Highlights
-
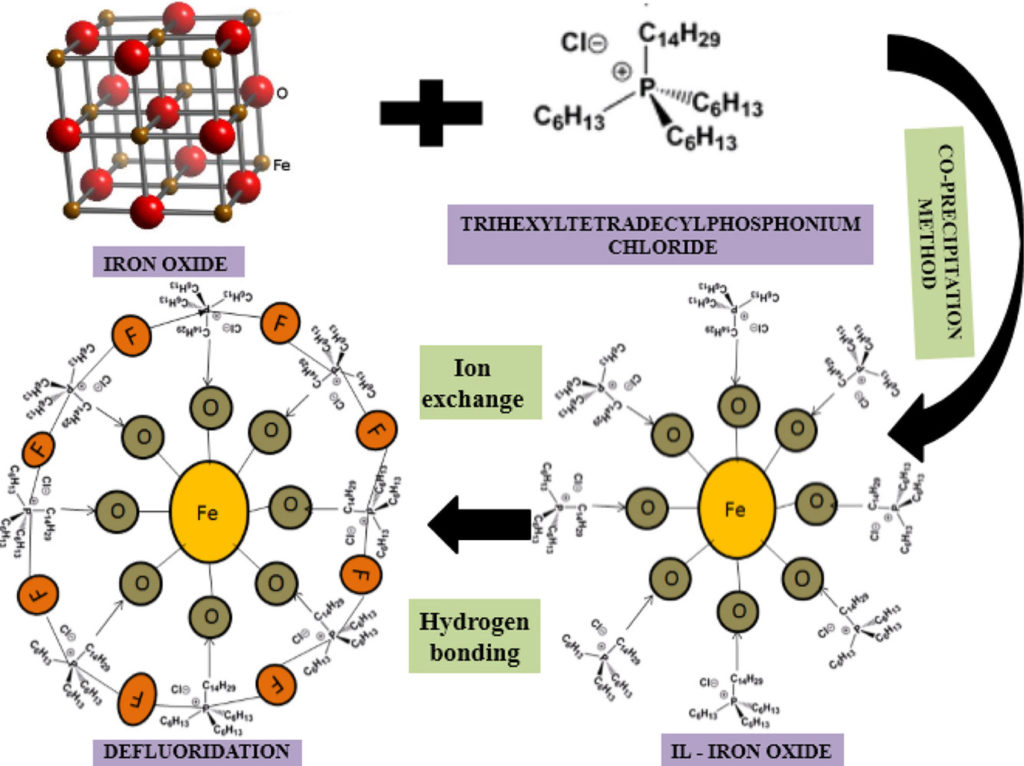
- IL-iron oxide NPs acts as an excellent adsorbent for fluoride adsorption since its ion pair attraction with the F–.
- Fluoride adsorption efficiency was achieved 96% in 30 min by IL-iron oxide NPs.
- Fluoride removal parameters on IL-iron oxide NPs have been optimized.
- Regeneration study showed 65% removal efficiency till 5th red Cycle.
Abstract
A promising ionic liquid modified iron oxide was synthesized with a co-precipitation method using trihexyltetradecylphosphonium chloride ionic liquid as a multi-functionalized material. The removal of fluoride was investigated by batch study. Ionic liquid with iron oxide nanoparticle characterization was conducted by Ultraviolet–visible spectroscopy, Fourier-transform infrared spectroscopy, Scanning electron microscope, X-ray powder diffraction, Raman spectroscopy, and Brunauer–Emmett–Teller. Batch adsorption was analyzed by varying parameters namely pH (8), contact time (30 min), adsorbent dosage (0.06 g/L), temperature (30 °C), initial concentration (10 mg/L), and stirring rate (400 rpm). The regression coefficient (R2) was the best fit at pseudo-second-order which was 0.99. The best isotherm model was Langmuir model which showed an adsorption capacity of 67.9 mg/g with maximum fluoride removal 96%. However, iron oxide modified with ionic liquid showed an efficient fluoride removal capacity and it can also be considered as an excellent adsorbent from aqueous solution.
Graphical abstract