Osteoarthritis, the number one cause of disability in the U.S., is a disease marked by a progressively debilitating stiffness and pain in the joints. The stiffness and pain results from degeneration in the joint cartilage, degeneration in the bone tissue underlying the joints, and bony overgrowth as well. (CDC 2011) The CDC estimates that over 27 million Americans have the condition.
It has long been observed that skeletal fluorosis (a bone disease caused by too much fluoride) can cause symptoms and degenerative changes that closely resemble osteoarthritis. While these arthritic effects were once considered to be confined to those with skeletal fluorosis, recent research shows that fluoride can cause osteoarthritis in the absence of traditionally defined fluorosis.
If conventional methods for detecting skeletal fluorosis continue to be used, many individuals with fluoride-induced osteoarthritis will not receive the correct diagnosis and treatment.
Symptoms and Bone Changes of Skeletal Fluorosis Can Closely Resemble Osteoarthritis
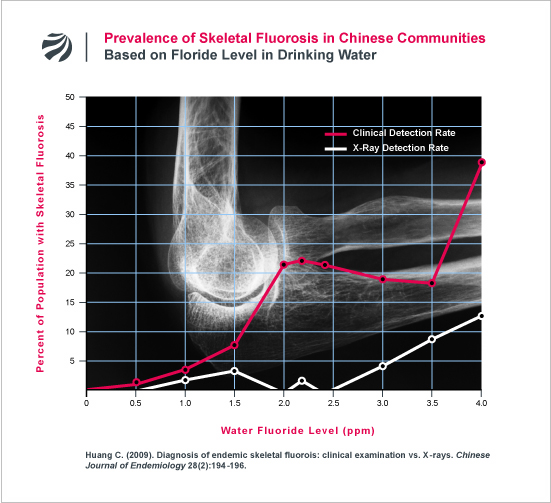
The symptoms of skeletal fluorosis (chronic joint pain and stiffness) mimic the symptoms of osteoarthritis. As an example of this, the following figure displays the findings from a recent Chinese study that investigated the prevalence of osteoarthritic symptoms in populations based on the level of fluoride in the drinking water:
It’s not just the symptoms of fluorosis that resemble osteoarthritis; the bone changes of fluorosis resemble osteoarthritis as well. Skeletal fluorosis causes bony outgrowths (i.e., osteophytes), degradation and calcification of cartilage, osteosclerosis, and reduced space between the joint — conditions common to osteoarthritis, including osteoarthritis of the spine (spondylosis). As noted in a recent study:
“because some of the early clinical symptoms resemble those of osteoarthritis, the first clinical phases of skeletal fluorosis could be easily misdiagnosed.” (Petrone 2011).
Fluoride Intake Can Cause Osteoarthritis
Not only can skeletal fluorosis produce bone changes that resemble osteoarthritis; it can cause osteoarthritis itself. (Luo 2012; Su 2012; Bao 2003; Savas 2001; Tartatovskaya 1995; Chen 1988; Xu 1987).
This fact was convincingly demonstrated in a recent, well-conducted study by a Chinese research group. (Bao 2003) In the study, the researchers x-rayed the right hands of adults living in a fluorosis area. They then compared these x-rays with the findings of a nearby non-fluorosis area and the findings of a nationwide study that they had previously conducted. The incidence of osteoarthritis in the fluorosis area was “remarkably higher” than in either the adjacent area or the nation as a whole. According to the researchers, “the osteoarthritis caused by fluorosis differs from ordinary osteoarthritis in severity rather than in nature.”
Fluoride Can Cause Osteoarthritis BEFORE Skeletal Fluorosis Is Evident
For years, U.S. health authorities have assumed that fluoride does not cause arthritic symptoms before the traditional bone changes of fluorosis are evident on x-ray. Recent research strongly suggests that this long-held assumption is in error.
Savas (2001)
Of particular significance is a study from Turkey which found strong evidence of a fluoride-osteoarthritis link in individuals who did not have telltale sign of skeletal fluorosis. (Savas 2001) According to the study, the most common radiological finding among the fluorosis patients was knee osteoarthritis — which was found in 66% of the 56 fluorosis patients examined. By contrast, only 3.6% of the fluorosis patients had axial osteosclerosis (i.e., hyperdense bone of the lower spine and pelvis), which is regarded by U.S. authorities to be the first radiological sign of fluorosis. Thus, many of the fluorosis patients had knee osteoarthritis without simultaneously showing the spinal bone changes that US authorities still deem necessary to warrant a diagnosis of skeletal fluorosis.
Tartatovskaya (1995)
Consistent with this Turkish study, a team of Russian researchers have found that fluoride-exposed individuals suffer a significantly elevated rate of osteoarthritis in the absence of radiologically detectable fluorosis in the spine. (Tartatovskaya 1995). The researchers, who were interested in determining whether fluoride exposure can exacerbate the wear and tear on joints from physical stress, examined two groups of mine workers with job activities that exposed them to significant vibration stress (e.g., drilling). Workers who were exposed to fluoride dusts were found to have a significantly higher rate of elbow osteoarthritis (48.7% vs. 12.9%) and spondylosis (83.3% vs. 41.1%) than the mine workers not exposed to fluoride dusts.
To test the veracity of these findings, the Russian researchers conducted animal studies where they subjected mice to the isolated and combined effects of vibration and fluoride exposure. As with the mine workers, the mice exposed to both vibration stress and fluoride experienced a greater frequency and earlier onset of degenerative joint changes than the mice exposed to either factor alone. The Russian researchers thus concluded that fluoride exposure can exacerbate the degenerative effect of physical stress on joints with or without the presence of radiologically detectable skeletal fluorosis.
Czerwinski (1988)
Consistent with the Turkish and Russian studies, a team of Polish researchers examined 2,258 fluoride-exposed workers in the aluminum industry and found high rates of arthritic effects in the absence of fluorosis bone changes. (Czerwinski 1988). Although the researchers could only detect fluorosis by x-ray in 1% of the workers, they found high rates of joint pain in the knee, hip, elbow, shoulder and lumbar spine, with the pains correlating to the duration of fluoride exposure. According to the researchers, “the only characteristic feature” of fluorosis is “multiple-joint involvement,” which “would differentiate fluorosis from monoarticular osteoarthritis, but unfortunately not from multiple-joint osteoarthritis or rheumatoid arthritis.”
Cao (2003)
Even among patients with crippling forms of fluorosis, degenerative joint damage can be the primary radiological finding, not osteosclerosis. In one study, for example, Chinese researchers found that 5 of 19 patients with crippling skeletal fluorosis “presented with mainly articular injury but relatively mild bone pathology.” (Cao 2003). The researchers termed this condition “fluorosis arthropathy.”
The Misdiagnosis Problem
The fact that fluoride can cause osteoarthritis in the joints prior to, and in the absence of, detectable osteosclerosis in the spine, highlights the difficulty of diagnosing fluorosis. Since many doctors continue to consider spinal osteosclerosis necessary for a diagnosis of skeletal fluorosis, many cases of fluoride-induced osteoarthritis (“secondary osteoarthritis”) will be misdiagnosed as “primary osteoarthritis,” thus depriving patients of the most effective treatment for the condition (reduction in fluoride exposure).
The difficulty of differentiating between osteoarthritis and early stage skeletal fluorosis was highlighted in a 1995 study by a team of Austrian doctors. (Roschger 1995). In the study, the doctors conducted x-rays of a woman who received high-dose fluoride treatment for 6 years as an experimental treatment for osteoporosis. After the woman sustained multiple spontaneous fractures, the doctors x-rayed her skeleton and measured her bone density. According to the doctors, “radiographs of the skeleton and bone scintigraphy showed degenerative osteoarthritis,” but none of the traditional signs of skeletal fluorosis. It wasn’t until the doctors performed a bone biopsy that the doctors were able to detect the presence of fluorosis. As the doctors noted, “Without bone biopsy we would have failed the correct diagnosis.” Based on this experience, the doctors concluded that “invasive investigation of the skeleton (bone biopsy, histomorphometry, BSEI plus SAXS) is the only diagnostic tool, when skeletal fluorosis is suspected.”
The problem with relying on x-rays to detect and prevent joint pathologies caused by fluoride is yet further demonstrated in the following figure. The figure presents the findings of a study from one of China’s most pre-eminent experts (Changqing Huang) on the diagnosis of skeletal fluorosis. As can be seen, many individuals diagnosed with fluorosis (based on detailed clinical examinations) did not have radiologically detectable fluorosis on x-ray.
References:
- Bao W, et al. (2003). Report of investigations on adult hand osteoarthritis in Fengjiabao Village, Asuo Village, and Qiancheng Village. Chinese Journal of Endemiology 22(6):517-18.
- Cao J, et al. (2003). Brick tea fluoride as a main source of adult fluorosis. Food and Chemical Toxicology 41(4):535-42.
- CDC (2011). Osteoarthritis. Available at: http://www.cdc.gov/arthritis/basics/osteoarthritis.htm
- Chen X. (1988): Radiological Analysis of Fluorotic Elbow Arthritis. Journal of Guiyang Medical College 13(2):303-305. (Article in Chinese translated into English by FAN)
- Czerwinski E, et al. (1988). Bone and joint pathology in fluoride-exposed workers. Archives of Environmental Health 43(5):340-3.
- Ge X, et al. (2006). Investigations on the occurrence of osteoarthritis in middle-aged and elderly persons in fluorosis-afflicted regions of Gaomi City with high fluoride concentration in drinking water. Preventive Medicine Tribune 12(1):57-58. (Article in Chinese translated into English by FAN)
- Luo R, et al. (2012). Total knee arthroplasty for the treatment of knee osteoarthritis caused by endemic skeletal fluorosis. Chinese Journal of Tissue Engineering Research.
- Petrone P, et al. (2011). Enduring Fluoride Health Hazard for the Vesuvius Area Population: The Case of AD 79 Herculaneum. PLoS ONE 6(6): e21085.
- Tartatovskaya LY, et al. (1995). Clinical and hygiene assessment of the combined effect on the body of vibration and fluorine. Noise and Vibration Bulletin 263-264.
- Roschger P, et al. (1995). Bone mineral structure after six years fluoride treatment investigated by backscattered electron imaging (BSEI) and small angle x-ray scattering (SAXS): a case report. Bone 16(3):407.
- Savas S, et al. (2001). Endemic fluorosis in Turkish patients: relationship with knee osteoarthritis. Rheumatology International 21(1):30-5.
- Su WM, et al. (2012). Total hip arthroplasty for the treatment of severe hip osteoarthritis due to fluorosis. Chinese Journal of Tissue Engineering Research 16(9):1543-1546.
- Xu JC, et al. (1987). X-ray findings and pathological basis of bone fluorosis. Chinese Medical Journal 100:8-16.
EXCERPTS: Skeletal fluorosis as a cause of osteoarthritis
“Now, total hip arthroplasty (THA) is one of the effective methods for the treatment of severe hip osteoarthritis due to fluorosis. OBJECTIVE: To investigate the strategies and efficacy of THA for the treatment of severe hip osteoarthritis due to fluorosis. METHODS: A total of five cases with severe hip osteoarthritis due to fluorosis were treated with THA using biological prosthesis. RESULTS AND CONCLUSION: All incisions were healed in one stage. Position of the prosthesis was good confirmed by X-rays observation at 1 week after operation. All the cases were followed-up for average of 13.8 months. Loosening and sinking of the prosthesis were not found during the follow-up. The average Harris scores were 83.6 and 87.8 points at postoperative 3 and 6 months respectively, which was improved as compared with preoperative score (38.4 points). Heterotopic ossification occurred in two cases, one was Brooker degree I and another was degree II. THA is an effective method to treat severe hip osteoarthritis due to fluorosis, which can significantly improve joint function and has few complications. Heterotopic ossification should be prevented after the operation.”
SOURCE: Su WM, et al. (2012). Total hip arthroplasty for the treatment of severe hip osteoarthritis due to fluorosis. Chinese Journal of Tissue Engineering Research 16(9):1543-1546.
“Knee osteoarthritis caused by endemic skeletal fluorosis is a complex and chronic systemic disease, which can cause the damage of surrounding bone and sclerotin of knee joint. . . . Nine patients who suffered from knee osteoarthritis caused by skeletal fluorosis . . . were treated with bilateral TKA [total knee arthroplasty].The clinical efficacy was evaluated according to the knee scoring system of United States Hospital for Special Surgery before arthroplasty and final follow-up after arthroplasty. Score results showed that 5 knees were excellent,3 knees were good and 1 knee was fair after knee arthroplasty. All patients have been improved in pain, function and range of motion. TKA is an effective method for the treatment of knee osteoarthritis caused by skeletal fluorosis.”
SOURCE: Luo R, et al. (2012). Total knee arthroplasty for the treatment of knee osteoarthritis caused by endemic skeletal fluorosis. Chinese Journal of Tissue Engineering Research. Available online at: http://en.cnki.com.cn/Article_en/CJFDTOTAL-XDKF201209015.htm
“A 47.2% overall occurrence of osteoarthritic-like lesions involving the joints of the appendicular skeleton appears particularly severe considering the mean age of 30.2 years (individuals >15-years-old). The coxofemoral, knee, sacroiliac, elbow, and sternoclavicular joints and pedal phalanges are the most noticeably affected anatomical districts . . . . [T]he concomitant aberrant growth of new bone, ligamentous calcification and osteosclerosis, along with osteoarthritic-like lesions and ankylosis of spine and appendicular joints, strongly suggest skeletal fluorosis.”
SOURCE: Petrone P, et al. (2011). Enduring Fluoride Health Hazard for the Vesuvius Area Population: The Case of AD 79 Herculaneum. PLoS ONE 6(6): e21085.
“Analysis of X-ray signs in 397 patients suffering from fluorosis revealed degenerative and dystrophic lumbar disorders in 94%, . . various changes (epicondylosis, periarthrosis and arthrosis deformans) in 91%. Locomotory disorders appeared systemic, multiple and symmetrical.”
SOURCE: Ornitsan E, et al. (2004). [Features of occupational fluorosis course]. Med Tr Prom Ekol. (12):27-9. (Translated from Russian to English by FAN)
“OBJECTIVE: To understand the relationship between fluorosis and adult osteoarthritis through the investigation of fluorosis-afflicted villages. METHODS: X-ray radiography of right hands was performed on 227 adults over the age of 40 from fluorosis-afflicted villages, and adult osteoarthritis was diagnosed using accumulated scores based on the obtained results. RESULTS: The identification rate of osteoarthritis in fluorosis-afflicted regions was 59.03%, and the mean accumulated score was 3.85, both significantly higher than those for the control population (identification rate was 23.6%, mean accumulated score was 0.72); patients with osteoarthritis caused by fluorosis accounted for a considerable portion of the osteoarthritis population. CONCLUSIONS: Fluorosis may lead to osteoarthritis, and also acts as a confounding factor of adult Kashin-Beck disease (KBD) in a portion of patients.”
Bao W, et al. (2003). Report of investigations on adult hand osteoarthritis in Fengjiabao Village, Asuo Village, and Qiancheng Village. Chinese Journal of Endemiology 22(6):517-18. (Translated from Chinese to English by FAN)
“”[A]rthopathy and arthritis affected a significant number of the (fluorosis) patients, resulting in functional disability. . . . The physical signs of brick-tea type skeletal fluorosis were elbow, shoulder and knee articular dysfunction, which was the most common pathology. X-ray examination revealed that the interosseous membrane ossification, tendon attachment calcification and articular degeneration were the causes of these functional disorders.”
SOURCE: Cao J, et al. (2003). Brick tea fluoride as a main source of adult fluorosis. Food and Chemical Toxicology 41(4):535-42.
“Endemic fluorosis may increase the severity of osteoarthritis in the knees. . . . Twelve patients (21.4%) with endemic fluorosis and eight control patients (20%) had grade 2 osteoarthritis, 16 patients (28.6%) with endemic fluorosis and three control patients (7.5%) had grade 3 osteoarthritis, and six patients (10.7%) with endemic fluorosis and one control patient (2.5%) had grade 4 osteoarthritis. The osteoarthritis severity was greater in the endemic fluorosis group. Osteophytes at the anterior and posterior parts of the tibial condyles, osteophytes at the superior margin of the patellar articular surface of the femur, and popliteal loose bodies were detected more frequently in the endemic fluorosis group than in controls. There was no difference between groups in the existence of osteophytes at the superior and inferior margins of the patella. However, these osteophytes were larger in endemic fluorosis patients than in controls (p = 0.001). Twenty-five (44.6%) patients with endemic fluorosis had polyp-like osteophytes at the medial non-weight-bearing margin of the femoral condyle. This kind of osteophyte was not detected in controls. . . . Further studies are needed in order to understand the exact mechanism of bone and cartilage changes in the knees of patients with endemic fluorosis.”
SOURCE: Savas S, et al. (2001). Endemic fluorosis in Turkish patients: relationship with knee osteoarthritis. Rheumatology International 21(1):30-5.
“Clinical and experimental data show, that osteoarthrosis deformans of the major joints (primarily elbow joint) must be regarded as one of the symptoms of fluorosis, when other intoxication signs are present.”
SOURCE: Semennikova TK, et al. (1992). [The clinical characteristics of the early stages of occupational fluorosis under the combined and joint action of production factors]. [Article in Russian]. Gig Tr Prof Zabol. (3):6-8. (Translated from Russian to English by FAN)
“A comparative study is presented of 378 workers with osteoarthrosis deformans (OD) contacting with fluorine compounds and in 106 patients with primary OD. The diagnostic criteria in these two categories of patients coincided. . . . In OD plus [industrial fluorosis] males prevailed, and were on average 10 years younger.”
SOURCE: Medvedeva VN. (1992). [The diagnosis and treatment of osteoarthrosis deformans in subjects in contact with fluorine compounds]. Lik Sprava. (8):76-8. (Translated from Russian to English by FAN)
“This article has reported the radiological signs in 109 cases of fluorotic elbow arthritis: sclerosis, irregularity and discontinuity of the articular surfaces; coarsened bone striation below the articular surface, trabecular coarsening and rarefaction, sparseness of trabeculae or density changes along with areas of cystic radiolucency. The author first suggested osteophyte formation in the lateral trochlear crest and ossification in the bilateral ligaments as the characteristic radiological signs for fluorotic elbow arthritis.”
Chen X. (1988): Radiological Analysis of Fluorotic Elbow Arthritis. Journal of Guiyang Medical College 13(2):303-305. (Translated from Chinese to English by FAN)
“Degenerative joint changes. Extensive degenerative changes may occur in patients with fluorine poisoning, resulting in osteoarthritis. Of the 146 cases, 107 (73%) had articular changes. Of these 107 cases, 12 were below 30 years of age and 2 were children. In the X-rays of dog’s limb big choints, changes resembling those in humans were seen.”
SOURCE: Xu JC, et al. (1987). X-ray findings and pathological basis of bone fluorosis. Chinese Medical Journal 100:8-16.
“Radiological investigation of skeletal fluorosis was carried out among the inhabitants from two areas where the fluoride content of water was high . . . . Degeneration of joints was a prominent feature.”
SOURCE: Lian ZC, Wu EH. (1986). Osteoporosis–an early radiographic sign of endemic fluorosis. Skeletal Radiology 15(5):350-3.
“Crippling fluorosis… is characterized by dense bones, exostoses, neurologic complications due to bony overgrowth, osteoarthritis, and ligamentous calcification.”
SOURCE: Riggs BL. (1983). Treatment of osteoporosis with sodium fluoride: An appraisal. Bone and Mineral Research Annual 2: 366-393.
“Even though extensive bone deformities may not be found on a large scale from fluoride in water at the 1 ppm concentration, some of the early signs of the disease, such as calcifications of ligaments, joint capsules, and muscle attachments, are likely to occur. Indeed these conditions are characteristic of osteoarthritis, in which the formation of microcrystals of apatite (known to be promoted by fluoride) has now been clearly demonstrated… For example, Pinet and Pinet described in detail X-ray changes encountered in skeletal fluorosis in North Africa that are in every respect identical with those present in the arthritic spine of the elderly elsewhere.”
SOURCE: Waldbott GL, Burgstahler AW, and McKinney HL. (1978). Fluoridation: The Great Dilemma. Coronado Press, Inc., Lawrence, Kansas.
“The investigation of a high incidence of arthritis in 21 dairy herds disclosed elevated fluorine levels in bone samples… There was a statistical correlation between a high incidence of damage to peri-articular structures, resulting in debility and loss of production, and elevated bone fluorine.”
SOURCE: Griffith-Jones W. (1977). Fluorosis in dairy cattle. The Veterinary Record 100: 84-89.
“Fluoric Arthropathies: Around joints, thick marginal osteophytes develop. In some instances, they grow to such an extent as to block joint movement (‘blocking arthrosis’). The joint block can also be induced by calcification of the periarticular ligament. The most common sites of articular involvement are the hips, the sacroiliac, elbow and knee joints. In older persons, the vetebral column is commonly affected. Advanced stages of the disease show atrophy and ulceration of joint cartilage.”
SOURCE: Soriano, M. (1968). Periostitis deformans due to wine fluorosis. Fluoride 1(2): 56-64.
“The ligamentous calcification [of skeletal fluorosis] is often periarticular and shows as osteoarthritis of the spine and hip joints as well as of the sacro-iliac joints.”
SOURCE: Kumar SP, Harper RA. (1963). Fluorosis in Aden. British Journal of Radiology 36: 497-502.
Mechanisms by which fluoride can cause osteoarthritis:
1) Degradation and Calcification of Cartilage:
“Fluoride mainly affects bone metabolism. However, cartilage is one of its deposition areas. In an animal study, it was shown that excessive fluoride ingestion caused necrosis of articular chrondocytes, ulcer formation, and articular calcification.”
SOURCE: Savas S, et al. (2001). Endemic fluorosis in Turkish patients: relationship with knee osteoarthritis. Rheumatology International 21(1):30-5.
“NaF [sodium fluoride] produces abnormal collagen fibres which provide an abnormal environment for calcification. The formation of defective collagen fibres during fluoride poisoning may explain the development of neobone in fluorosis. NaF is suggested to interfere with maturation of collegen fibres by exerting an adverse effect on cross-link precursors.”
SOURCE: Sharma YD. (1982). Effect of sodium fluoride on collage cross-link precursors. Toxicology Letters 10:97-100.
“It is concluded that fluoride, ingested in excessive amounts, increases the solubility and degradation of collagen and reduces the collagen biosynthesis and cross-links. Therefore, the matured tissue collagen fibers would be abnormal due to inadequate cross-linking.”
SOURCE: Sharma YD. (1982). Variations in the metabolism and maturation of collagen after fluoride ingestion. Biochimica et Biophysica Acta 715:137-41.
“[I]t is postulated that fluoride activates the calcification of cartilage.”
SOURCE: Bang S, et al. (1985). Distribution of fluoride in calcified cartilage of a fluoride-treated osteoporotic patient. Bone 6: 207-210.
“In general, the metabolic patterns of osteoblasts, ameloblasts, odontoblasts, and chrondoblasts are sufficiently similar so that disturbances of cartilage might be expected… To date, any osteoarthritis observed in fluoride-treated cattle has been regarded as an unrelated process. However, excessive remodeling of the subchondral plate and cancellous end of the bone, such as occurs in osteofluorosis, will eventually lead to remodeling of the articular cartilage. Excessive cartilage remodeling leads to osteoarthritis of normal joints. Therefore, both the mechanical effects of fluoride induced remodeling and the direct action of fluoride on cartilage cells might alter cartilage. The fluoride levels and remodeling circumstances necessary to produce cartilage alteration in cattle – if it occurs – remain to be established.”
SOURCE: Johnson LC. (1965). Histogenesis and mechanisms in the development of osteofluorosis. In: H.C.Hodge and F.A.Smith, eds : Fluorine chemistry, Vol. 4. New York, N.Y., Academic press (1965) 424-441.
2) Enlargement of Bone Tissue (Osteosclerosis & Bony Outgrowths):
“Osteoarthritis, osteonecrosis, and fractures can occur [in skeletal fluorosis] as a result of the marked bone hardness. Some bone changes can imitate a seronegative arthritis.”
SOURCE: Kalia LV, et al. (2010). Thoracic myelopathy from coincident fluorosis and epidural lipomatosis. Can. J. Neurol. Sci. 37:276-78.
“Studies directed toward correlating fluoride-induced increases in bone density with non-fatal diseases, such as osteoarthritis, should be conducted.”
SOURCE: Prival MJ. (1972). Fluorides and human health. Center for Science in the Public Interest, Washington D.C.