Highlights
-
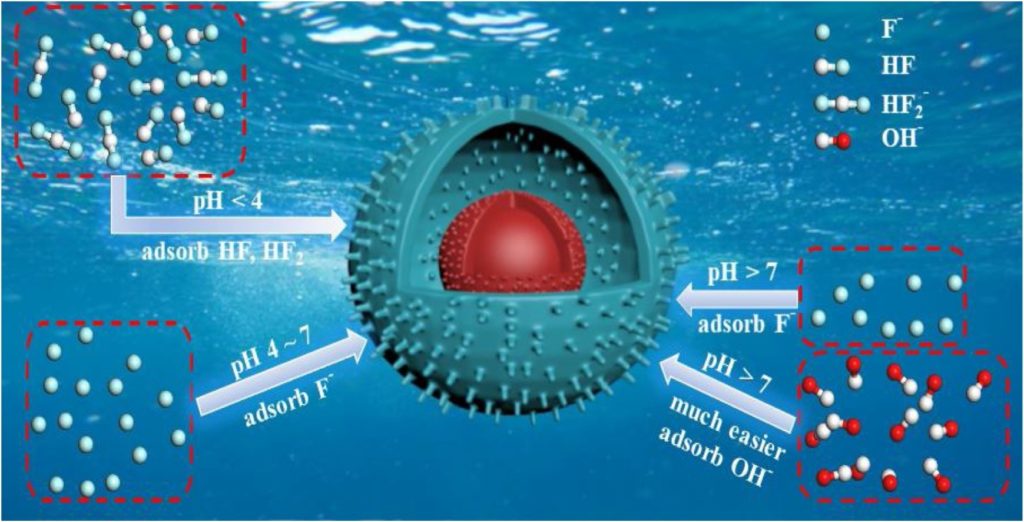
- The species of F–, HF, HF2– played more important role at different pH.
- The lattice plane (0 0 2) of core-shell Boehmite was the dominant adsorptive face.
- The Al, O and H sites were adsorptive sites with fluoride species at different pH.
- The OH– had the competitive effect of adsorption on adsorptive sites at higher pH.
Abstract
Many kinds of adsorbents have been developed for removing fluoride from water. However, the unclear actual mechanism of fluoride adsorption greatly restricts the structural design and application of novel adsorbents. Based on the understanding of the interaction between hydroxyl and fluoride, a novel core-shell nanostructure of boehmite was synthesized via an in-situ-induced assembly for removing fluoride. The formed polycrystalline boehmite (Y-AlOOH) nanostructure significantly enhances adsorption performance. The transformation of fluoride forms (including F–, HF, HF2–) is closely related to the solution property. The acidic solution is more favorable, mainly because of the conversion of HF (pyrazine) and HF2– (the bifluoride ion) with a strong hydrogen bond effect from fluoride (F–) with pH < 3.18. The lattice plane of (0 0 2) belongs to the dominant face for removing fluoride in this structure. According to the experimental and theoretical calculation, strong bonding of Al, O and H sites with fluoride species (F–, HF, HF2–) in acidic solution are demonstrated, but not in alkaline solution due to OH– interference. The possible mechanism of fluoride adsorption on boehmite (AlOOH) structures is proposed. Our findings show a new potential prospect of structural designing for novel fluoride adsorbent.
Graphical abstract