Abstract
It is critical to determine the mechanism underlying fluoride (F)-induced damage of the testes to develop appropriate strategies for monitoring and intervention. In the present study, exposure to 50 mg/L sodium fluoride (NaF) for 90 days damaged the normal structure of the testes and quality of the sperm, particularly the spermatocytes, and triggered overexpression of human antigen R (Elavl1/HuR) according to western blotting and immunofluorescence. Furthermore, 0.5 mM NaF exposure for 24 h exposure increased the proportion of apoptosis and expression of caspase-3 and caspase-9 in mouse spermatocytes (GC-2spd cell line), whereas inhibition of HuR reduced apoptosis and the expression of caspase-3 and caspase-9. Additionally, inhibition of HuR alleviated F-induced autophagy based on observation of the autophagy bodies, detection of autophagy activity, and analysis of the expression of the LC3II/LC3I and p62 proteins. These results reveal that excessive F can lead to overexpression of HuR, resulting in high levels of apoptosis and autophagy in spermatocytes. These findings improve the understanding of the mechanisms underlying F-induced male reproductive toxicity, and HuR may be explored as a treatment target for certain conditions.
Graphical abstract
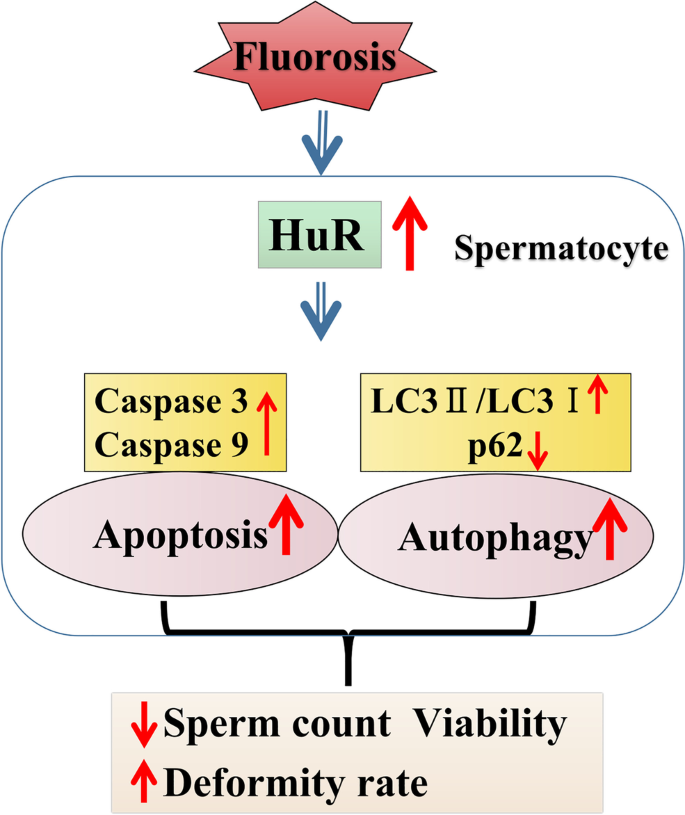
Excessive fluoride can induce overexpression of HuR in testis and result in excessive apoptosis and autophagy in spermatocytes as well as male reproductive damage, such as a decreased sperm count, decreased sperm motility, and increased deformity rate.
Excerpt:
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to our research continues, such as exploring the mechanism of up regulation of HuR caused by fluorine. Hence, our original data will not be public temporarily. [REASON(S) WHY DATA ARE NOT PUBLIC] but are available from the corresponding author on reasonable request.
References
-
Buzalaf MAR (2018) Review of fluoride intake and appropriateness of current guidelines. Adv Dent Res 29:157–166. https://doi.org/10.1177/0022034517750850
-
Dhar V, Bhatnagar M (2009) Physiology and toxicity of fluoride. Indian J Dent Res 20:350–355. https://doi.org/10.4103/0970-9290.57379
-
Rezaee T, Bouxsein ML, Karim L (2020) Increasing fluoride content deteriorates rat bone mechanical properties. Bone 136:115369. https://doi.org/10.1016/j.bone.2020.115369
-
Herath H, Kawakami T, Tafu M (2018) The extremely high adsorption capacity of fluoride by chicken bone char (CBC) in defluoridation of drinking water in relation to its finer particle size for better human health. Healthcare (Basel). https://doi.org/10.3390/healthcare6040123
-
Peckham S, Awofeso N (2014) Water fluoridation: a critical review of the physiological effects of ingested fluoride as a public health intervention. ScientificWorldJournal 2014:293019. https://doi.org/10.1155/2014/293019
-
Liang C, He Y, Liu Y, Gao Y, Han Y, Li X, Zhao Y, Wang J, Zhang J (2020) Fluoride exposure alters the ultra-structure of sperm flagellum via reducing key protein expressions in testis. Chemosphere 246:125772. https://doi.org/10.1016/j.chemosphere.2019.125772
-
Zhang S, Niu Q, Gao H, Ma R, Lei R, Zhang C, Xia T, Li P, Xu C, Wang C, Chen J, Dong L, Zhao Q, Wang A (2016) Excessive apoptosis and defective autophagy contribute to developmental testicular toxicity induced by fluoride. Environ Pollut 212:97–104. https://doi.org/10.1016/j.envpol.2016.01.059
-
Adelakun SA, Akintunde OW, Ogunlade B (2020) Fluoride-induced testicular degeneration and sperm quality deteriorations: salutary role of Cyperus esculentus tubers (tiger nut) extract in animal model. Rev Int Androl. https://doi.org/10.1016/j.androl.2020.01.003
-
Li Y, Min C, Zhao Y, Wang J, Wang J (2021) Effects of fluoride on PIWI-interacting RNA expression profiling in testis of mice. Chemosphere 269:128727. https://doi.org/10.1016/j.chemosphere.2020.128727
-
Raghunath A, Jeyabaskar D, Sundarraj K, Panneerselvam L, Perumal E (2016) In silico prediction of microRNAs on fluoride induced sperm toxicity in mice. Food Chem Toxicol 98:34–49. https://doi.org/10.1016/j.fct.2016.03.005
-
Huo M, Han H, Sun Z, Lu Z, Yao X, Wang S, Wang J (2016) Role of IL-17 pathways in immune privilege: a RNA deep sequencing analysis of the mice testis exposure to fluoride. Sci Rep 6:32173. https://doi.org/10.1038/srep32173
-
Mirisis AA, Carew TJ (2019) The ELAV family of RNA-binding proteins in synaptic plasticity and long-term memory. Neurobiol Learn Mem 161:143–148. https://doi.org/10.1016/j.nlm.2019.04.007
-
Rossi G, Placidi M, Castellini C, Rea F, D’Andrea S, Alonso GL, Gravina GL, Tatone C, Di Emidio G, D’Alessandro AM (2021) Crocetin mitigates irradiation injury in an in vitro model of the pubertal testis: focus on biological effects and molecular mechanisms. Molecules. https://doi.org/10.3390/molecules26061676
-
Wei L, Lee S, Majumdar S, Zhang B, Sanfilippo P, Joseph B, Miura P, Soller M, Lai EC (2020) Overlapping activities of ELAV/Hu family RNA binding proteins specify the extended neuronal 3’ UTR landscape in Drosophila. Mol Cell 80:140-155.e146. https://doi.org/10.1016/j.molcel.2020.09.007
-
Brun LR, Pera LI, Rigalli A (2010) Bone morphometry and differences in bone fluorine containing compounds in rats treated with NaF and MFP. Biomed Pharmacother 64:1–6. https://doi.org/10.1016/j.biopha.2008.10.009
-
de Carvalho JG, Cestari TM, de Oliveira RC, Buzalaf MA (2008) Fluoride effects on ectopic bone formation in young and old rats. Methods Find Exp Clin Pharmacol 30:287–294. https://doi.org/10.1358/mf.2008.30.4.1159651
-
Im K, Mareninov S, Diaz MFP, Yong WH (1897) An introduction to performing immunofluorescence staining. Methods Mol Biol 2019:299–311. https://doi.org/10.1007/978-1-4939-8935-5_26
-
Chen J, Chen T, Zhu Y, Li Y, Zhang Y, Wang Y, Li X, Xie X, Wang J, Huang M, Sun X, Ke Y (2019) circPTN sponges miR-145-5p/miR-330-5p to promote proliferation and stemness in glioma. J Exp Clin Cancer Res 38:398. https://doi.org/10.1186/s13046-019-1376-8
-
Srivastava S, Flora SJS (2020) Fluoride in drinking water and skeletal fluorosis: a review of the global impact, Curr Environ. Health Rep 7:140–146. https://doi.org/10.1007/s40572-020-00270-9
-
Islam MS, Mostafa MG (2021) Meta-analysis and risk assessment of fluoride contamination in groundwater. Water Environ Res 93:1194–1216. https://doi.org/10.1002/wer.1508
-
Hao P, Ma X, Cheng X, Ba Y, Zhu J, Cui L (2010) Effect of fluoride on human hypothalamus-hypophysis-testis axis hormones. Wei Sheng Yan Jiu 39:53–55
-
Zhao WP, Wang HW, Liu J, Tan PP, Luo XL, Zhu SQ, Chen XL, Zhou BH (2018) Positive PCNA and Ki-67 expression in the testis correlates with spermatogenesis dysfunction in fluoride-treated rats. Biol Trace Elem Res 186:489–497. https://doi.org/10.1007/s12011-018-1338-6
-
Feng D, Huang H, Yang Y, Yan T, Jin Y, Cheng X, Cui L (2015) Ameliorative effects of N-acetylcysteine on fluoride-induced oxidative stress and DNA damage in male rats’ testis. Mutat Res Genet Toxicol Environ Mutagen 792:35–45. https://doi.org/10.1016/j.mrgentox.2015.09.004
-
Zeiger E, Shelby MD, Witt KL (1993) Genetic toxicity of fluoride. Environ Mol Mutagen 21:309–318. https://doi.org/10.1002/em.2850210402
-
He X, Sun Z, Manthari RK, Wu P, Wang J (2018) Fluoride altered rat’s blood testis barrier by affecting the F-actin via IL-1?. Chemosphere 211:826–833. https://doi.org/10.1016/j.chemosphere.2018.08.009
-
Dai P, Wang X, Gou LT, Li ZT, Wen Z, Chen ZG, Hua MM, Zhong A, Wang L, Su H, Wan H, Qian K, Liao L, Li J, Tian B, Li D, Fu XD, Shi HJ, Zhou Y, Liu MF (2019) A translation-activating function of MIWI/piRNA during mouse spermiogenesis. Cell 179:1566-1581.e1516. https://doi.org/10.1016/j.cell.2019.11.022
-
Zaharieva E, Haussmann IU, Bräuer U, Soller M (2015) Concentration and localization of coexpressed ELAV/Hu proteins control specificity of mRNA processing. Mol Cell Biol 35:3104–3115. https://doi.org/10.1128/mcb.00473-15
-
Yang C, Yao C, Ji Z, Zhao L, Chen H, Li P, Tian R, Zhi E, Huang Y, Han X, Hong Y, Zhou Z, Li Z (2021) RNA-binding protein ELAVL2 plays post-transcriptional roles in the regulation of spermatogonia proliferation and apoptosis. Cell Prolif 54:e13098. https://doi.org/10.1111/cpr.13098
-
Lee WY, Lee R, Park HJ, Do JT, Park C, Kim JH, Jhun H, Lee JH, Hur T, Song H (2017) Characterization of male germ cell markers in canine testis. Anim Reprod Sci 182:1–8. https://doi.org/10.1016/j.anireprosci.2017.01.002
-
Chi MN, Auriol J, Jégou B, Kontoyiannis DL, Turner JM, de Rooij DG, Morello D (2011) The RNA-binding protein ELAVL1/HuR is essential for mouse spermatogenesis, acting both at meiotic and postmeiotic stages. Mol Biol Cell 22:2875–2885. https://doi.org/10.1091/mbc.E11-03-0212
-
Grammatikakis I, Abdelmohsen K, Gorospe M (2017) Posttranslational control of HuR function, Wiley Interdiscip Rev RNA. https://doi.org/10.1002/wrna.1372
-
Schultz CW, Preet R, Dhir T, Dixon DA, Brody JR (2020) Understanding and targeting the disease-related RNA binding protein human antigen R (HuR). Wiley Interdiscip Rev RNA 11:e1581. https://doi.org/10.1002/wrna.1581
-
Li XX, Xiao L, Chung HK, Ma XX, Liu X, Song JL, Jin CZ, Rao JN, Gorospe M, Wang JY (2020) Interaction between HuR and circPABPN1 modulates autophagy in the intestinal epithelium by altering ATG16L1 translation, Mol Cell Biol. https://doi.org/10.1128/mcb.00492-19
-
Benoit RM, Meisner NC, Kallen J, Graff P, Hemmig R, Cèbe R, Ostermeier C, Widmer H, Auer M (2010) The X-ray crystal structure of the first RNA recognition motif and site-directed mutagenesis suggest a possible HuR redox sensing mechanism. J Mol Biol 397:1231–1244. https://doi.org/10.1016/j.jmb.2010.02.043
-
Karginov FV (2019) HuR controls apoptosis and activation response without effects on cytokine 3’ UTRs. RNA Biol 16:686–695. https://doi.org/10.1080/15476286.2019.1582954
-
Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH (2013) Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol 14:32. https://doi.org/10.1186/1471-2121-14-32
-
Noori AR, Tashakor A, Nikkhah M, Eriksson LA, Hosseinkhani S, Fearnhead HO (2021) Loss of WD2 subdomain of Apaf-1 forms an apoptosome structure which blocks activation of caspase-3 and caspase-9. Biochimie 180:23–29. https://doi.org/10.1016/j.biochi.2020.10.013
-
Zhang S, Jiang C, Liu H, Guan Z, Zeng Q, Zhang C, Lei R, Xia T, Gao H, Yang L, Chen Y, Wu X, Zhang X, Cui Y, Yu L, Wang Z, Wang A (2013) Fluoride-elicited developmental testicular toxicity in rats: roles of endoplasmic reticulum stress and inflammatory response. Toxicol Appl Pharmacol 271:206–215. https://doi.org/10.1016/j.taap.2013.04.033
-
Wei Q, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L (2018) A mini review of fluoride-induced apoptotic pathways. Environ Sci Pollut Res Int 25:33926–33935. https://doi.org/10.1007/s11356-018-3406-z
-
Campanario S, Ramírez-Pardo I, Hong X, Isern J, Muñoz-Cánoves P (2020) Assessing autophagy in muscle stem cells. Front Cell Dev Biol 8:620409. https://doi.org/10.3389/fcell.2020.620409
-
Marafon BB, Pinto AP, da Rocha AL, Rovina RL, Pauli JR, De Moura LP, Cintra DE, Ropelle ER, Da Silva ASR (2020) Hepatic LC3 II/I ratio is not modulated in exercised mice. Physiol Res 69:1103–1111. https://doi.org/10.33549/physiolres.934441
-
Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, Hoshii T, Hirao A, Takagi K, Mizushima T, Motohashi H, Lee MS, Yoshimori T, Tanaka K, Yamamoto M, Komatsu M (2013) Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell 51:618–631. https://doi.org/10.1016/j.molcel.2013.08.003
-
Zhang J, Zhu Y, Shi Y, Han Y, Liang C, Feng Z, Zheng H, Eng M, Wang J (2017) Fluoride-induced autophagy via the regulation of phosphorylation of mammalian targets of rapamycin in mice Leydig cells. J Agric Food Chem 65:8966–8976. https://doi.org/10.1021/acs.jafc.7b03822
-
Feng Z, Liang C, Manthari RK, Wang C, Zhang J (2019) Effects of fluoride on autophagy in mouse sertoli cells. Biol Trace Elem Res 187:499–505. https://doi.org/10.1007/s12011-018-1405-z
-
Liang C, Gao Y, He Y, Han Y, Manthari RK, Tikka C, Chen C, Wang J, Zhang J (2020) Fluoride induced mitochondrial impairment and PINK1-mediated mitophagy in Leydig cells of mice: in vivo and in vitro studies. Environ Pollut 256:113438. https://doi.org/10.1016/j.envpol.2019.113438
-
Li X, Meng L, Wang F, Hu X, Yu Y (2019) Sodium fluoride induces apoptosis and autophagy via the endoplasmic reticulum stress pathway in MC3T3-E1 osteoblastic cells. Mol Cell Biochem 454:77–85. https://doi.org/10.1007/s11010-018-3454-1
-
Chen Q, Li Z, Xu Z, Chen C, Wang J, Zhu J, Dong Z (2021) miR-378d is involved in the regulation of apoptosis and autophagy of and E(2) secretion from cultured ovarian granular cells treated by sodium fluoride. Biol Trace Elem Res 199:4119–4128. https://doi.org/10.1007/s12011-020-02524-x
-
Wang Y, Li A, Mehmood K, Hussain R, Abbas RZ, Javed MT, Chang YF, Hu L, Pan J, Li Y, Shi L, Tang Z, Zhang H (2021) Long-term exposure to the fluoride blocks the development of chondrocytes in the ducks: the molecular mechanism of fluoride regulating autophagy and apoptosis. Ecotoxicol Environ Saf 217:112225. https://doi.org/10.1016/j.ecoenv.2021.112225
Acknowledgements
The authors thank Center for Health Aging (Changzhi Medical College) and College Central Laboratory (Changzhi Medical College) for valuable help in our experiment.
Funding
This work is supported by Doctoral research fund of Changzhi Medical College (No. BS202006).
