Abstract
Sodium fluoride (NaF) is widely used in clinical dentistry. However, the administration of high or low concentrations of NaF has various functions in different tissues. Understanding the mechanisms of the different effects of NaF will help to optimize its use in clinical applications. Studies of NaF and epithelial cells, osteoblasts, osteoclasts, and periodontal cells have suggested the significant roles of fluoride treatment. In this review, we summarize recent studies on the biphasic functions of NaF that are related to both soft and hard periodontal tissues, multiple diseases, and clinical dentistry.
Keywords: fluoride; epithelial cells; mesenchymal stem cells; osteoblasts; osteoclasts; periodontal disease; miRNA
*Full-text study online at https://www.mdpi.com/1422-0067/23/2/962/htm
Excerpt:
1. Introduction
Fluoride is well known for its use in the treatment of dental caries, either systemically or topically [1]. Fluoride intake (such as in drinking water, fluoridated toothpaste, or fluoride supplements) is the cornerstone to preventing dental caries in adults and children. Fluoride prevents dental caries by slowing down the demineralization of enamel, which is caused by the interaction between dental plaque and dental hard tissues. Fluoride may inhibit tooth decay by 40–60% by co-precipitating calcium and phosphate ions and by enhancing the precipitation of fluoridated apatite. Fluoride is also found deposited as calcium fluoride in dental plaque, which helps to prevent dental caries [2]. However, a high concentration of fluoride alters the mineralization process and can cause hypomineralization or fluorosis [3]. It has been demonstrated that a concentration of fluoride between 1.6 and 1.8 ppm in the drinking water is the risk threshold for dental fluorosis [4]. Osteoporosis and osteosclerosis are induced by high concentrations of sodium fluoride (NaF) in the drinking water [5]. Genotoxicity has been found to be related to oxidative stress or cell cycle disturbances induced by high concentrations of fluoride [6].
The effects of fluoride are biphasic. Exposing human dental pulp cells to a low concentration of NaF (25 uM–50 uM) stimulated proliferation and differentiation, while a concentration of NaF at 50 uM to 100 uM stimulated proliferation, differentiation, and collagen synthesis in human osteoblastic osteosarcoma cells [7]. Myoblasts exposed to low concentrations of NaF promoted proliferation, which could be regarded as a muscle enhancing factor, and high concentrations elevated the expression of muscle atrophy-related genes, myostatin and atrogin-1, reactive oxygen species (ROS), and inflammatory factors that accelerate skeletal muscle atrophy [8]. Fluoride can alter the bone mineral metabolism and can affect the homeostasis of bone formation and resorption [9]. Early studies demonstrated that the intake of fluoridated water can inhibit hydrocortisone-induced bone resorption and parathormone-induced alveolar bone resorption [10,11]. Using fluoride as a therapeutic agent to treat postmenopausal osteoporosis in adults has also been investigated [12].
High and low concentrations of fluoride result in toxic or tolerated dosages, respectively, making it vital to determine the concentration of fluoride intake. Various signaling pathways can alter cellular or metabolic actions in response to either high or low concentrations of fluoride. This review focuses on the various effects induced by high and low concentrations of fluoride.
2. Fluoride and Epithelial Cells
2.1. High Concentration Fluoride and Epithelial Cells
A high concentration of NaF (millimolar level) induces endoplasmic reticulum stress, apoptosis, and DNA fragmentation and interferes with enamel proteinases [17]. Exposure to a high concentration of fluoride results in enamel hypomineralization, also known as enamel fluorosis [18]. The severity of enamel fluorosis increases depending on the volume of fluoride intake and the duration of fluoride exposure [18]. Fluoride decreases the abilities of proteinases such as MMP-20 and KLK4 that degrade enamel matrix proteins, and as a result, inhibits crystal growth [19]. Alternatively, the intake of fluoride lowers the pH, which inhibits the synthesis and secretion of KLK4 [20]. One study reported that excessive fluoride results in hypomineralization through the reduced expression of Forkhead box O1 (FOXO1) in dental epithelial cells [21]. FOXO1 is reduced after exposure to excessive fluoride, which might affect the expressions of KLK4 and amelotin (AMTN), which ultimately results in enamel fluorosis. One mM fluoride was found to be cytotoxic and induced apoptosis in human gingival epithelial cells. Treatment with 2 mM NaF disturbed the gene network accompanied by endoplasmic reticulum stress [22].
2.2. Low Concentration Fluoride and Epithelial Cells
Studies have shown that fluoride has biphasic effects on epithelial ameloblast-lineage cells at different concentrations. Reduced proliferation was observed at a fluoride concentration higher than 1 mM; however, lower concentrations of fluoride, e.g., around 16 uM, promoted the proliferation of epithelial ameloblast-lineage cells [23].
During gingival wound healing, human epithelial cells cover the wound, a process termed re-epithelialization. The process of wound healing includes epithelial cell proliferation and migration as well as the synthesis and deposition of extracellular matrix components [24]. Extracellular matrix proteins such as fibronectin and laminin-5 are expressed by migrating keratinocytes during wound healing. To understand the characteristics of gingival epithelial cells responding to fluoride, human primary epithelial cells were treated with NaF to characterize their effects on cellular physiology. Cell proliferation peaked at a concentration of 50 uM of NaF, and a higher concentration of NaF (at the millimolar level) reduced proliferation. Cells treated with 50 uM of NaF showed significantly more motility than non-treated cells in vitro. qRT-PCR analyses showed increased mRNA expression levels of fibronectin and laminin-5 in cells treated with 50 uM of NaF [25].
2.3. Fluoride and Epithelial–Mesenchymal Interactions
Epithelial–mesenchymal interactions (EMT) are important for the development of ectodermal organs and for tissue regeneration and wound healing, including cellular events such as cell adhesion, proliferation, differentiation, and maturation [26]. Homeostasis, inflammation, migration and proliferation, and remodeling are the four major processes of wound healing [27]. Fibroblast growth factor 2 (FGF2) and its receptor, fibroblast growth factor receptor 2 (FGFR2), are crucial for proliferation, migration, and protease production in epithelial cells [28]. FGF2 is associated with EMT by inducing mesenchymal characteristics in epithelial cells [29]. Fibroblast growth factor 7 (FGF7) is essential for epithelial morphogenesis [30]. Twist family BHLH transcription factor 1 (Twist1) is a critical mediator for EMT [31]. One study demonstrated that treatment with 50 uM of NaF induces the expression of FGF2 and FGF7, and Twist1 is also upregulated in vivo. It was also found that the untreated group had more persistent wounds in mice [32].
3. Fluoride and Bone Marrow Mesenchymal Stem Cells (BMMSCs)
BMMSCs present in the bone marrow are self-renewing precursor cells that have the multipotency to differentiate into osteoblasts, chondroblasts, adipoblasts, and stromal cells [33]. Upon bone injuries, BMMSCs differentiate into osteoblasts and release growth factors during wound healing [34]. Consistent with other studies, fluoride also has dual effects on BMMSCs. A low concentration of NaF (50 or 500 uM) enhanced the proliferation of BMMSCs, while a high concentration of NaF (5 mM) reduced their proliferation. Furthermore, BMMSCs showed elevated motility and migration when compared with the control group. Treatment with 50 uM of NaF upregulated the expression of fibronectin and vimentin, which induced the Runt-related transcription factor (Runx2), induced osteoblast differentiation, and increased the secretion of osteocalcin (OCN) [35]. Treatment with a higher concentration of NaF induced cytotoxicity, DNA damage, and oxidative stress in BMMSCs [36]. Studies have indicated that fluoride-induced cytotoxicity depends on the concentration and the duration of fluoride exposure [37]. High concentrations of NaF (2 mM and above) result in oxidative stress and decreased viability and proliferation via the phosphor-c-Jun N-terminal kinase (JNK) pathway. In dental pulp stem cells, a low concentration of NaF promotes osteo/odontogenic differentiation via the PI3K/Akt pathway [38]. In addition, a low concentration of NAF (0.5 mM) is the optimal concentration to regulate the osteo/odontogenic differentiation of stem cells from apical papilla [39]. When exposed to mouse embryonic stem cells, a high concentration of NaF (over 1 mM) induced ROS and reduced DNA synthesis, which resulted in apoptosis through a JNK-dependent pathway [40].
4. Fluoride and Bone Metabolism
Bone homeostasis is maintained through bone formation by osteoblasts and bone resorption by osteoclasts [41]. The activity of osteoblasts and osteoclasts is critical for bone maintenance and remodeling. The process is regulated by many factors, such as sex hormones, parathyroid hormones, and calcitonin, as well as various growth factors and cytokines [42]. Osteoblasts and osteoclasts also can control each other’s formation, differentiation, apoptosis through multiple pathways via cytokines, extracellular proteins, and transcription factors [43]. Bone diseases such as osteoporosis are caused by dysfunctional bone remodeling or the disruption of the homeostasis maintained by osteoblasts and osteoclasts [43].
Both positive and negative effects of fluoride on bones and teeth have been well established [44,45]. Fluoride treatment increases the proliferation of osteoblasts and inhibits the function of osteoclasts [46]. Trace amounts of fluoride have been used to treat osteoporosis, vertebral fractures, and bone loss in patients [47], whereas excessive fluoride results in skeletal fluorosis [48]. Long-term exposure to fluoride can result in skeletal and dental fluorosis [49]. Thus, a better understanding of the effects of different concentrations of NaF on bone homeostasis will help to gain more insights into the usage of fluoride.
4.1. Fluoride and Osteoblasts
OCN and Osteopontin (OPN) are non-collagenous proteins secreted by osteoblasts that serve as markers for osteoblast maturation [50]. OCN promotes bone formation and regulates mineralization in the bone matrix, which has a complex regulatory network [51]. OPN is an extracellular matrix protein that has multiple functions associated with bone structuring and destruction in osseous tissues [52]. The transcription factor Runx2 induces the expression of osteoblastic genes including OCN and OPN [53]. Runx2 is expressed at different stages of osteoblasts (pre-osteoblasts, and immature and mature osteoblasts) and is essential for bone formation and osteoblastic differentiation [54]. Osterix (OSX), a zinc-finger containing a transcription factor, is also a downstream target of Runx2 [55]. Together with Runx2, OSX regulates the differentiation of pre-osteoblasts into mature osteoblasts and osteocytes, and the roles of OSX in maintaining bone homeostasis have been well studied [37]. One study showed that NaF treatment affects calcium homeostasis and transcription factor expression [56]. A low concentration of NaF induced Runx2 and OSX while a high concentration of NaF reduced their expression both at the mRNA and protein levels. Treatment of MC3T3-E1 cells with 50 or 500 uM of NaF enhanced their proliferation, alkaline phosphatase (ALP) activity, and extracellular matrix mineralization, as well as their expression of bone-related genes (Runx2, OSX, OCN, and OPN) [57]. Different concentrations of NaF were also found to differentially affect the expression of bone mineralized regulator proteins, such as OCN, OPN, osteonectin (ON), and bone sialoprotein (BSP) in bone marrow stromal cells. A higher concentration of NaF (10-5 M) decreased the level of the proteins while a lower concentration (10-7 M) increased them [44].
Various cellular mechanisms, including the Mitogen-activated protein kinase (MAPK) pathway, are proposed to function in bone formation during fluoride treatment [58]. It is well established that a low concentration of NaF (micromolar levels) regulates tyrosine kinase and ALP activity to increase osteoblast proliferation [59,60]. Fluoride treatment of osteoblasts is also biphasic in that low concentrations promote proliferation, whereas higher concentrations result in signs of weakened osteoblast activity [61]. Long periods of fluoride exposure reduce the expression of bcl-2 family proteins, which promotes apoptosis in osteoblastic cells [62]. Fluoride induces mitochondrial respiratory chain complex abnormal expressions, which in turn cause oxidative stress and result in apoptosis [63]. High concentrations of NaF also regulate the proliferation and cell cycle of the p16 gene methylation during the development of skeletal fluorosis [64]. The schematic diagram shown in Figure 1 summarizes the functions of NaF in osteoblasts.
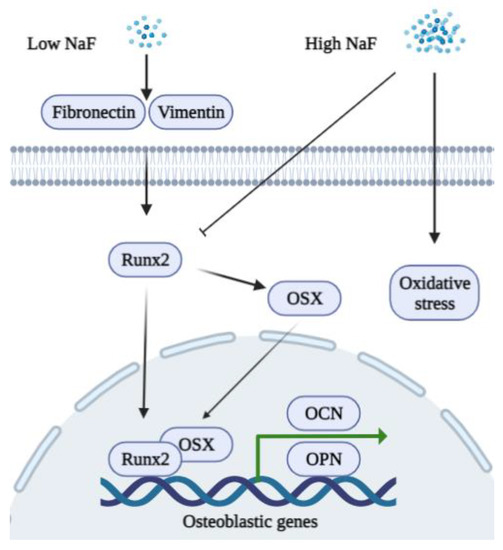
Figure 1. A schematic diagram of the biphasic functions of fluoride in osteoblasts. A low concentration of NaF stimulates the expression of fibronectin, vimentin, Runx2, and OSX to promote the expression of osteoblastic genes (OCN and OPN). A high concentration of NaF inhibits Runx2 and induces oxidative stress in osteoblasts.
4.2. Fluoride and Osteoclasts
Osteoprotegerin (OPG) is a soluble cytokine receptor of the tumor necrosis factor (TNF) receptor family that binds to the OPG ligand [65]. The receptor activator of nuclear factor kappa-B ligand (RANKL) is also a member of the TNF superfamily, which binds to RANK on target cells. OPG/RANKL/RANK is a key signaling pathway in bone metabolism. The overexpression of OPG alters osteoclast differentiation, and recombinant OPG impedes ovariectomy-induced bone loss in rats [66]. Recombinant OPG binds to OPG ligand on BMMSCs, thereby inhibiting osteoclast differentiation [67]. RANKL is an essential cytokine that regulates osteoclastogenesis and bone resorption [68]. In response to RANKL activation, the nuclear factor of activated T cells 1 (NFATc1) regulates the terminal differentiation of osteoclasts, and NFATc1-deficient embryonic stem cells were unable to differentiate into osteoclasts [69]. NFATc1 participates in the transcription of the genes involved in heart/valve septum formation, angiogenesis, T cell proliferation, and osteoclast formation. NFATc1 binds to the promoter of genes associated with bone resorption, including cathepsin K and matrix metalloproteinase 9 (MMP-9), thus inducing their gene expression [70].
Low concentrations of NaF (micromolar level) have little influence on the viability of BMMSCs and significantly downregulate both mRNA and the protein expression levels of NFATc1 in rat osteoclasts, which result in a reduction in the levels of cathepsin K and the attenuation of bone destruction [71]. Treatment with 0.5 mM to 1 mM of NaF inhibits the activity of osteoclasts in vitro [72]. Studies suggest that fluoride can act on matrix proteinases such as Metalloproteinases-2 and -9, thus inhibiting the matrix degradation [73,74]. Low concentrations of fluoride can possibly regulate osteoclasts via the B lymphocyte-induced maturation protein-1 (Blimp1)/B cell lymphoma 6 (Bcl6) axis which is a critical signaling pathway that regulates osteoclast differentiation and bone homeostasis [75]. On the other hand, the stimulation of NaF exhibits a U-shaped curve in a dose-dependent manner [76]. High concentrations of NaF induce RANKL and decrease OPG, thus increasing osteocyte-driven osteoclastogenesis via the RANK-JNK-NFATc1 signaling pathway [77]. A recent study found that osteoclasts demonstrate the most sensitivity to high concentrations of NaF with respect to other bone cell types and that fluoride exposure induces apoptosis via the Transforming growth factor (TGF)-? signaling pathway [78]. A 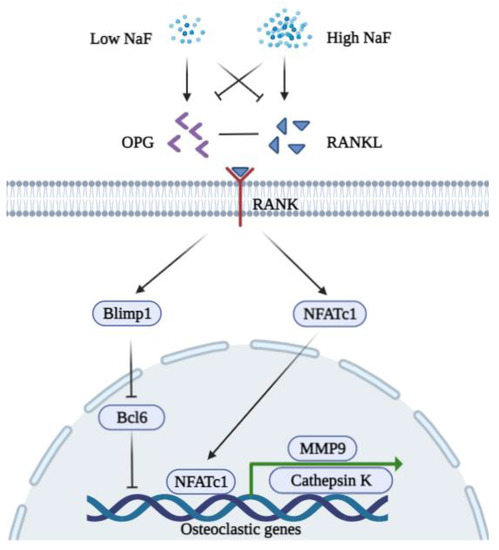 Figure 2. A schematic diagram of the biphasic functions of fluoride in osteoclasts. A low concentration of NaF inhibits the expression of RANKL, NFATc1, MMP9, and Cathepsin K but induces OPG to bind RANKL. A low concentration of NaF is possibly associated with Blimp1/Bcl6 to repress the expression of osteoclastic genes (MMP9 and Cathepsin K). A high concentration of NaF decreases the expression of OPG while inducing RANKL to bind RANK. Induced NFATc1 translocate into the nucleus to promote osteoclastogenesis.
Figure 2. A schematic diagram of the biphasic functions of fluoride in osteoclasts. A low concentration of NaF inhibits the expression of RANKL, NFATc1, MMP9, and Cathepsin K but induces OPG to bind RANKL. A low concentration of NaF is possibly associated with Blimp1/Bcl6 to repress the expression of osteoclastic genes (MMP9 and Cathepsin K). A high concentration of NaF decreases the expression of OPG while inducing RANKL to bind RANK. Induced NFATc1 translocate into the nucleus to promote osteoclastogenesis.
5. Fluoride and Periodontal Diseases
Periodontal diseases are induced by bacterial biofilms caused by Gram-negative anaerobic bacteria [79]. Dental plaque formed after bacteria colonize teeth stimulates the host’s inflammation responses in gingival connective tissues [80,81]. Chronic periodontitis is mediated by interactions between the host and pathogens, which result in the apical migration of epithelial attachment and ultimately the destruction of connective tissue and alveolar bone [82,83].
A low concentration of NaF had anti-inflammatory effects that decreased the expression of IL-1B, IL-8, and TNF-a in human gingival fibroblasts [84]. A recent study demonstrated that NaF has an antibacterial activity against P. gingivalis in a concentration-dependent manner. A model of rat periodontitis treated with 500 uM of NaF in drinking water showed attenuated alveolar bone resorption and reduced levels of interleukins-6 and -8. The expression of NFATc1 was also reduced in osteoclasts and cathepsin K [71]. Another study found that treatment with NaF could reduce P. gingivalis-induced inflammation and bone loss in rat periodontitis tissues and significantly reduce the infiltration of polymorphonuclear leukocytes (PMN) [57].
Diabetes mellitus (DM) is associated with a reduction in insulin production or relative changes that increase glucose levels in the blood during insulin activity [85]. Hyperglycemia induced by increased glucose affects many tissues and organs, such as the kidneys, nerves, blood vessels, and periodontal tissues. Periodontitis is the sixth complication of DM, and they share various pathogenic mechanisms; however, the exact mechanism by which diabetes is associated with periodontitis is not fully understood [86]. One study reported that periodontitis models with diabetes suffer more alveolar bone loss than those without diabetes [87].
Insulin-like growth factors (IGFs) are involved in the development and growth of B cells that are important for B cells’ mitogenic action and inhibit B cell apoptosis [88]. The fetal and adult pancreas can produce IGFs and their binding receptors. The overexpression of IGF-2 causes apoptosis in islets and islet hyperplasia, while increased B cell mass elevates IGF-1 expression. The elevation of IGF-1 or mediators in the IFG signaling pathway protects B cells from apoptosis, thus preventing diabetes in mice [89,90,91].
IGFs are autocrine/paracrine factors that regulate the proliferation, differentiation and functions of osteoblasts, osteocytes, and osteoclasts [92,93]. Fluoride is possibly associated with IFG-1 to enhance osteogenic cell proliferation through protein tyrosine phosphorylation [12,59,61]. A recent study confirmed that the expression of IGF-1, IFG-2, IFG-1R, and IGF-2R increased after NaF treatment, while the expression of TNF-?, IL-1?, RANKL, and cathepsin K decreased. These effects contributed to attenuated alveolar bone resorption caused by a low concentration of NaF [94].
Autophagy is a cellular process that eliminates damaged proteins and organelles, which is important for maintaining cellular homeostasis [95]. Autophagy-related 5 (ATG5) is essential for forming autophagosomes, and ATG5 knockout in mice results in the dysfunction of autophagy and cell death [96]. ATG5 stimulates light chain 3 (LC3)-I and LC3-II and subsequently generates autophagosomes [97]. Beclin-1 helps to recruit ATG proteins and activates downstream genes [98]. Beclin-1 is regarded as an inter-mediator between apoptosis and autophagy, and the dysfunction of autophagy could result in excessive apoptosis [99].
Accumulating evidence suggests that autophagy has an important role in periodontal inflammation [100]. One study demonstrated that a high concentration of NaF induces the apoptosis of human cementoblasts through the inhibition of autophagy. Autophagy-related genes ATG5 and Beclin-1 were suppressed, resulting in alveolar bone resorption [101].
A high concentration of NaF induces oxidative stress, leading to the dysfunction of mitochondria and the stimulation of cell apoptosis [102,103]. Autophagy may serve a protective role in response to fluoride-induced oxidative damage, and the impairment of autophagy results in excessive apoptosis [104,105]. A low concentration of NaF facilitated the expression of osteo-/odontogenic markers of apical papilla cells via the enhanced autophagy pathway [39]. Figure 3 summarizes the biphasic functions of fluoride in periodontal inflammation.
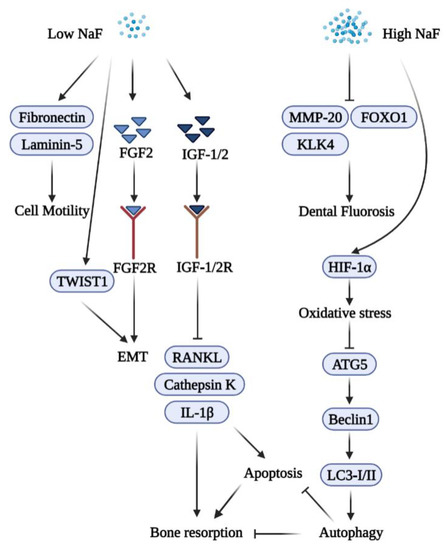
6. Fluoride and microRNA
Excessive NaF intake ameliorates dental and skeletal fluorosis. miR-124 and miR-155 are reported to be critical players in fluorosis biology [106]. The recent finding of increased cyclinD1 expression due to miR-486-3p made a significant contribution to understanding the mechanism of skeletal fluorosis [107]. Finding that cyclinD1 is also a direct target of miR-4755-5p, and fluoride exposure suppresses miR-4755-5p and induces cyclinD1 protein in osteoblasts, thus shedding new light upon fluorosis treatment [108]. miR-200c-3p is also considered a potential biomarker for skeletal fluorosis [109]. NaF treatment upregulates miR-200c-3p expression via the BMP4/Smad signaling pathway [109]. NaF exposure mainly affects the Notch, Wnt, hedgehog, and TGF-beta signaling pathways in the osteoblasts [110]. miR-122-5p targets CDK4 protein in NaF-treated human osteoblasts, suggesting its involvement in the etiology of skeletal fluorosis [111]. Skeletal fluorosis may alter the expression level of 10 candidate miRNAs in mouse osteoblast cells through numerous signaling molecules, including autophagy [112]. Female reproductive malfunction could be a result of excessive NaF intake, and the microRNAs (miRNAs) play a substantial role in the regulation of reproduction. A recent study demonstrated that miR-378d was inversely correlated with the autophagy markers under NaF exposure on ovarian cells [113]. Fluoride exposure alters the miR-34 family to orchestrate the downstream signaling molecules in sperm [114]. PIWI-interacting RNAs (piRNAs) are explicitly suggested as candidate biomarkers for fluoride-induced testicular toxicity [115]. Perinatal fluoride exposure can lead to learning and memory problems in mouse offspring, at least in part by the alteration of miR-124 and miR-132 upregulation and their targets [116].
*Read the full study at http://fluoridealert.org/wp-content/uploads/wang-2022.pdf
